
ŅŅ“¼ŹĒÖŲŅŖµÄÓŠ»ś»Æ¹¤ŌĮĻ£¬æÉÓÉŅŅĻ©ĘųĻąÖ±½ÓĖ®ŗĻ·ØÉś²ś»ņ¼ä½ÓĖ®ŗĻ·ØÉś²ś”£»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)¼ä½ÓĖ®ŗĻ·ØŹĒÖøĻČ½«ŅŅĻ©ÓėÅØĮņĖį·“Ӧɜ³ÉĮņĖįĒāŅŅõ„(C2H5OSO3H)£¬ŌŁĖ®½āÉś³ÉŅŅ“¼”£Š“³öĻąÓ¦·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ________________________________________”£
(2)ŅŃÖŖ£ŗ
¼×“¼µÄĶŃĖ®·“Ó¦
2CH3OH(g)===CH3OCH3(g)£«H2O(g)
¦¤H1£½£23.9 kJ”¤mol£1
¼×“¼ÖĘĻ©ĢžµÄ·“Ó¦
2CH3OH(g)===C2H4(g)£«2H2O(g)
¦¤H2£½£29.1 kJ”¤mol£1
ŅŅ“¼µÄŅģ¹¹»Æ·“Ó¦””C2H5OH(g)===CH3OCH3(g)
¦¤H3£½£«50.7 kJ”¤mol£1
ŌņŅŅĻ©ĘųĻąÖ±½ÓĖ®ŗĻ·“Ó¦C2H4(g)£«H2O(g)===C2H5OH(g)µÄ¦¤H________kJ”¤mol£1”£Óė¼ä½ÓĖ®ŗĻ·ØĻą±Č£¬ĘųĻąÖ±½ÓĖ®ŗĻ·ØµÄÓŵćŹĒ____________________________________”£
(3)ČēĶ¼ĖłŹ¾ĪŖĘųĻąÖ±½ÓĖ®ŗĻ·ØÖŠŅŅĻ©µÄĘ½ŗā×Ŗ»ÆĀŹÓėĪĀ¶Č”¢Ń¹ĒæµÄ¹ŲĻµ[ĘäÖŠn(H2O)”Ćn(C2H4)£½1”Ć1]”£
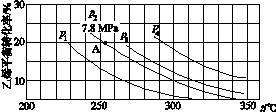
¢ŁĮŠŹ½¼ĘĖćŅŅĻ©Ė®ŗĻÖĘŅŅ“¼·“Ó¦ŌŚĶ¼ÖŠAµćµÄĘ½ŗā³£ŹżKp£½____________________(ÓĆĘ½ŗā·ÖŃ¹“śĢęĘ½ŗāÅØ¶Č¼ĘĖć£¬·ÖŃ¹£½×ÜŃ¹”ĮĪļÖŹµÄĮæ·ÖŹż)”£
¢ŚĶ¼ÖŠŃ¹Ēæ(p1”¢p2”¢p3”¢p4)µÄ“óŠ”Ė³ŠņĪŖ____£¬ĄķÓÉŹĒ___________________”£
¢ŪĘųĻąÖ±½ÓĖ®ŗĻ·Ø³£²ÉÓĆµÄ¹¤ŅÕĢõ¼žĪŖ£ŗĮ×Ėį/¹čŌåĶĮĪŖ“߻ƼĮ£¬·“Ó¦ĪĀ¶Č290 ”ę”¢Ń¹Ēæ6.9 MPa£¬n(H2O)”Ćn(C2H4)£½0.6”Ć1”£ŅŅĻ©µÄ×Ŗ»ÆĀŹĪŖ5%£¬ČōŅŖ½ųŅ»²½ĢįøßŅŅĻ©×Ŗ»ÆĀŹ£¬³żĮĖæÉŅŌŹŹµ±øı䷓ӦĪĀ¶ČŗĶŃ¹ĒæĶā£¬»¹æɲÉČ”µÄ“ėŹ©ÓŠ________________________________________________________________________”¢
________________________________________________________________________ӣ
(1)C2H4£«H2SO4ØD”śC2H5OSO3H”¢C2H5OSO3H£«H2OØD”śC2H5OH£«H2SO4””(2)£45.5””ĪŪČ¾Š””¢øÆŹ“ŠŌŠ”µČ””(3)¢Ł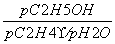 £½
£½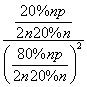 £½
£½
 £½0.07(MPa)£1””¢Śp1<p2<p3<p4””·“Ó¦·Ö×ÓŹż¼õÉŁ£¬ĻąĶ¬ĪĀ¶ČĻĀ£¬Ń¹ĒæÉżøßŅŅĻ©×Ŗ»ÆĀŹĢįøß
£½0.07(MPa)£1””¢Śp1<p2<p3<p4””·“Ó¦·Ö×ÓŹż¼õÉŁ£¬ĻąĶ¬ĪĀ¶ČĻĀ£¬Ń¹ĒæÉżøßŅŅĻ©×Ŗ»ÆĀŹĢįøß
¢Ū½«²śĪļŅŅ“¼Ņŗ»ÆŅĘČ„””Ōö¼Ón(H2O)”Ćn(C2H4)±Č
[½āĪö] (1)øł¾ŻĢāÖŠŠÅĻ¢æÉŠ“³öÓÉŅŅĻ©ÓėÅØĮņĖį¼ä½ÓĖ®ŗĻ·ØÖĘŅŅ“¼µÄ·“Ó¦ĪŖC2H4£«H2SO4ØD”śC2H5OSO3H ŗĶC2H5OSO3H£«H2OØD”śC2H5OH£«H2SO4”£(2)øł¾ŻøĒĖ¹¶ØĀÉ¢Ł£¢Ś£¢ŪµĆ£ŗC2H4(g)£«H2O(g)===C2H5OH(g)””¦¤H£½£45.5 kJ”¤mol£1”£¼ä½ÓĖ®ŗĻ·ØÖŠÓƵ½ÅØĮņĖįµČĒæøÆŹ“ŠŌĪļÖŹ£¬ÓėĘäĻą±ČÖ±½ÓĖ®ŗĻ·Ø¾ßÓŠĪŪČ¾Š””¢øÆŹ“ŠŌŠ”µČÓÅµć”£(3)¢ŁÉčĘšŹ¼Ź±C2H4ŗĶH2O(g)µÄĪļÖŹµÄĮæ¾łĪŖn£¬øł¾ŻC2H4µÄ×Ŗ»ÆĀŹĪŖ20%£¬ŌņĘ½ŗāŹ±C2H4”¢H2O(g)ŗĶC2H5OHµÄĪļÖŹµÄĮæ·Ö±šĪŖ80%n”¢80%nŗĶ20%n£¬ŌņKp£½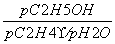 £½
£½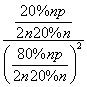 £½
£½ £½0.07(MPa)£1”£¢ŚŌö“óŃ¹Ēæ£¬Ę½ŗā½«ÕżĻņŅĘ¶Æ£¬ÄÜĢįøßC2H4µÄ×Ŗ»ÆĀŹ£¬¼“Ń¹Ēæp1£¼p2£¼p3£¼p4”£¢ŪĪŖĮĖŹ¹Ę½ŗāÕżĻņŅĘ¶Æ£¬»¹æÉŅŌ½«ŅŅ“¼Ņŗ»Æ¼°Ź±·ÖĄė£¬»ņŌö“ón (H2O)£ŗn (C2H4) Ö®±ČµČ“ėŹ©”£
£½0.07(MPa)£1”£¢ŚŌö“óŃ¹Ēæ£¬Ę½ŗā½«ÕżĻņŅĘ¶Æ£¬ÄÜĢįøßC2H4µÄ×Ŗ»ÆĀŹ£¬¼“Ń¹Ēæp1£¼p2£¼p3£¼p4”£¢ŪĪŖĮĖŹ¹Ę½ŗāÕżĻņŅĘ¶Æ£¬»¹æÉŅŌ½«ŅŅ“¼Ņŗ»Æ¼°Ź±·ÖĄė£¬»ņŌö“ón (H2O)£ŗn (C2H4) Ö®±ČµČ“ėŹ©”£


 ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø
ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŌĖÓĆĻą¹Ų»ÆѧÖŖŹ¶½ųŠŠÅŠ¶Ļ£¬ĻĀĮŠ½įĀŪ“ķĪóµÄŹĒ(””””)
A£®Ä³ĪüČČ·“Ó¦ÄÜ×Ō·¢½ųŠŠ£¬Ņņ“ĖøĆ·“Ó¦ŹĒģŲŌö·“Ó¦
B£®NH4FĖ®ČÜŅŗÖŠŗ¬ÓŠHF£¬Ņņ“ĖNH4FČÜŅŗ²»ÄÜ“ę·ÅÓŚ²£Į§ŹŌ¼ĮĘæÖŠ
C£®æÉČ¼±łÖ÷ŅŖŹĒ¼×ĶéÓėĖ®ŌŚµĶĪĀøßŃ¹ĻĀŠĪ³ÉµÄĖ®ŗĻĪļ¾§Ģ壬Ņņ“ĖæÉ“ęŌŚÓŚŗ£µ×
D£®Ōö“ó·“Ó¦ĪļÅضČæɼÓæģ·“Ó¦ĖŁĀŹ£¬Ņņ“ĖÓĆÅØĮņĖįÓėĢś·“Ó¦ÄÜŌö“óÉś³ÉH2µÄĖŁĀŹ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Ģś¼°Ęä»ÆŗĻĪļÓėÉś²ś”¢Éś»ī¹ŲĻµĆÜĒŠ”£
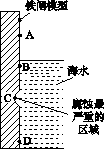
(1)ĻĀĶ¼ŹĒŹµŃéŹŅŃŠ¾æŗ£Ė®¶ŌĢśÕ¢²»Ķ¬²æĪ»øÆŹ“ĒéæöµÄĘŹĆęŹ¾ŅāĶ¼”£
¢ŁøƵē»ÆøÆŹ“³ĘĪŖ________”£
¢ŚĶ¼ÖŠA”¢B”¢C”¢DĖÄøöĒųÓņ£¬Éś³ÉĢśŠā×ī¶ąµÄŹĒ________(Ģī×ÖÄø)”£
(2)ÓĆ·ĻĢśĘ¤ÖĘČ”Ģśŗģ(Fe2O3)µÄ²æ·ÖĮ÷³ĢŹ¾ŅāĶ¼ČēĻĀ£ŗ
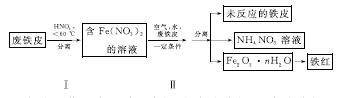
¢Ł²½Öč¢ńČōĪĀ¶Č¹żøߣ¬½«µ¼ÖĀĻõĖį·Ö½ā”£ĻõĖį·Ö½āµÄ»Æѧ·½³ĢŹ½ĪŖ______________________________”£
¢Ś²½Öč¢ņÖŠ·¢Éś·“Ó¦£ŗ4Fe(NO3)2£«O2£«(2n£«4)H2O===2Fe2O3”¤nH2O£«8HNO3£¬·“Ó¦²śÉśµÄHNO3ÓÖ½«·ĻĢśĘ¤ÖŠµÄĢś×Ŗ»ÆĪŖFe(NO3)2£¬øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ____________________________”£
¢ŪÉĻŹöÉś²śĮ÷³ĢÖŠ£¬ÄÜĢåĻÖ”°ĀĢÉ«»Æѧ”±Ė¼ĻėµÄŹĒ______(ČĪŠ“Ņ»Ļī)”£
(3)ŅŃÖŖt ”ꏱ£¬·“Ó¦FeO(s)£«CO(g)Fe(s)£«CO2(g)µÄĘ½ŗā³£ŹżK£½0.25”£
¢Łt ”ꏱ£¬·“Ó¦“ļµ½Ę½ŗāŹ±n(CO)”Ćn(CO2)£½________”£
¢ŚČōŌŚ1 LĆܱÕČŻĘ÷ÖŠ¼ÓČė0.02 mol FeO(s)£¬²¢ĶØČėx mol CO, t ”ꏱ·“Ó¦“ļµ½Ę½ŗā”£“ĖŹ±FeO(s)×Ŗ»ÆĀŹĪŖ50%£¬Ōņx£½________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĄūÓĆĢģČ»ĘųæÉÖʵĆŅŌH2”¢COµČĪŖÖ÷ŅŖ×é³ÉµÄ¹¤ŅµŌĮĻŗĻ³ÉĘų£¬·“Ó¦ĪŖCH4(g)£«H2O(g)
 CO(g)£«3H2(g)”£
CO(g)£«3H2(g)”£
(1)¼×ĶéÓėĖ®ÕōĘų·“Ó¦£¬±»Ńõ»ÆµÄŌŖĖŲŹĒ____________£¬µ±Éś³É±ź×¼×“æöĻĀ35.84 LŗĻ³ÉĘųŹ±×ŖŅʵē×ÓµÄĪļÖŹµÄĮæŹĒ________”£
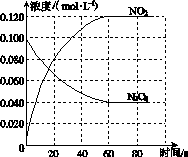
(2)½«2 mol CH4ŗĶ5 mol H2O(g)ĶØČėČŻ»żĪŖ100 LµÄ·“Ó¦ŹŅ£¬CH4µÄĘ½ŗā×Ŗ»ÆĀŹÓėĪĀ¶Č”¢Ń¹ĒæµÄ¹ŲĻµČēĶ¼K235ĖłŹ¾”£
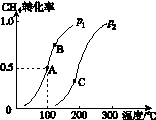
Ķ¼K235
¢ŁČō“ļµ½AµćĖłŠčµÄŹ±¼äĪŖ5 min£¬Ōņv(H2)£½________________________________________________________________________£¬
100 ”ęŹ±Ę½ŗā³£ŹżK£½____________________”£
¢ŚĶ¼ÖŠµÄp1______p2(Ģī”°<”±”°>”±»ņ”°£½”±)£¬A”¢B”¢CČżµćµÄĘ½ŗā³£ŹżKA”¢KB”¢KCµÄ“󊔹ŲĻµŹĒ________________________________________________________________________”£
(3)ŗĻ³ÉĘųÓĆÓŚŗĻ³É°±ĘųŹ±Šč³żČ„CO£¬·¢Éś·“Ó¦CO(g)£«H2O(g) 
 CO2(g)£«H2(g)””¦¤H<0£¬ĻĀĮŠ“ėŹ©ÖŠÄÜŹ¹
CO2(g)£«H2(g)””¦¤H<0£¬ĻĀĮŠ“ėŹ©ÖŠÄÜŹ¹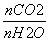 Ōö“óµÄŹĒ________(Ń”Ģī±ąŗÅ)”£
Ōö“óµÄŹĒ________(Ń”Ģī±ąŗÅ)”£
A£®½µµĶĪĀ¶Č
B£®ŗćĪĀŗćČŻĻĀ³äČėHe(g)
C£®½«H2“ÓĢåĻµÖŠ·ÖĄė
D£®ŌŁĶØČėŅ»¶ØĮæµÄĖ®ÕōĘų
æÉÓĆĢ¼Ėį¼ŲČÜŅŗĪüŹÕÉś³ÉµÄCO2£¬³£ĪĀĻĀpH£½10µÄĢ¼Ėį¼ŲČÜŅŗÖŠÓÉĖ®µēĄėµÄOH£µÄĪļÖŹµÄĮæÅضČĪŖ________________________________________________________________________£¬
³£ĪĀĻĀ£¬0.1 mol”¤L£1 KHCO3ČÜŅŗÖŠpH>8£¬ŌņČÜŅŗÖŠc(H2CO3)________c(CO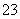 )(Ģī”°>”±”°£½”±»ņ”°<”±)”£
)(Ģī”°>”±”°£½”±»ņ”°<”±)”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŌŚČŻ»żĪŖ1.00 LµÄČŻĘ÷ÖŠ£¬ĶØČėŅ»¶ØĮæµÄN2O4£¬·¢Éś·“Ó¦N2O4(g)2NO2(g)£¬ĖęĪĀ¶ČÉżøߣ¬»ģŗĻĘųĢåµÄŃÕÉ«±äÉī”£
»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)·“Ó¦µÄ¦¤H________0(Ģī”°“óÓŚ”±»ņ”°Š”ÓŚ”±)£»100 ”ꏱ£¬ĢåĻµÖŠø÷ĪļÖŹÅضČĖꏱ¼ä±ä»ÆČēĶ¼ĖłŹ¾”£ŌŚ0”«60 sŹ±¶Ī£¬·“Ó¦ĖŁĀŹv(N2O4)ĪŖ________mol”¤L£1”¤s£1£»·“Ó¦µÄĘ½ŗā³£ŹżK1ĪŖ________”£
(2)100 ”ꏱ“ļĘ½ŗāŗó£¬øı䷓ӦĪĀ¶ČĪŖT£¬c(N2O4)ŅŌ0.002 0 mol”¤L£1”¤s£1µÄĘ½¾łĖŁĀŹ½µµĶ£¬¾10 sÓÖ“ļµ½Ę½ŗā”£
¢ŁT________100 ”ę(Ģī”°“óÓŚ”±»ņ”°Š”ÓŚ”±)£¬ÅŠ¶ĻĄķÓÉŹĒ____________________________”£
¢ŚĮŠŹ½¼ĘĖćĪĀ¶ČTŹ±·“Ó¦µÄĘ½ŗā³£ŹżK2£ŗ_______________________________________
________________________________________________________________________ӣ
(3)ĪĀ¶ČTŹ±·“Ó¦“ļĘ½ŗāŗ󣬽«·“ӦȯĘ÷µÄČŻ»ż¼õÉŁŅ»°ė£¬Ę½ŗāĻņ________(Ģī”°Õż·“Ó¦”±»ņ”°Äę·“Ó¦”±)·½ĻņŅĘ¶Æ£¬ÅŠ¶ĻĄķÓÉŹĒ__________________________________________________
________________________________________________________________________ӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŅŃÖŖ£ŗ
C(s)£«O2(g)===CO2(g)””¦¤H1
CO2(g)£«C(s)===2CO(g)””¦¤H2
2CO(g)£«O2(g)===2CO2(g)””¦¤H3
4Fe(s)£«3O2(g)===2Fe2O3(s)””¦¤H4
3CO(g)£«Fe2O3(s)===3CO2(g)£«2Fe(s)””¦¤H5
ĻĀĮŠ¹ŲÓŚÉĻŹö·“Ó¦ģŹ±äµÄÅŠ¶ĻÕżČ·µÄŹĒ(””””)
A£®¦¤H1>0£¬¦¤H3<0 B£®¦¤H2>0£¬¦¤H4>0
C£®¦¤H1£½¦¤H2£«¦¤H3 D£®¦¤H3£½¦¤H4£«¦¤H5
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
MgAgClµē³ŲŹĒŅ»ÖÖÄܱ»ŗ£Ė®¼¤»īµÄŅ»“ĪŠŌÖü±øµē³Ų£¬µē³Ų·“Ó¦·½³ĢŹ½ĪŖ2AgCl£« Mg === Mg2£«£« 2Ag £«2Cl£”£ÓŠ¹ŲøƵē³ŲµÄĖµ·ØÕżČ·µÄŹĒ(””””)
A£®MgĪŖµē³ŲµÄÕż¼«
B£®øŗ¼«·“Ó¦ĪŖAgCl£«e£===Ag£«Cl£
C£®²»Äܱ»KCl ČÜŅŗ¼¤»ī
D£®æÉÓĆÓŚŗ£ÉĻÓ¦¼±ÕÕĆ÷¹©µē
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
½«ŗ£Ė®µ»ÆÓėÅØŗ£Ė®×ŹŌ“»Æ½įŗĻĘšĄ“ŹĒ×ŪŗĻĄūÓĆŗ£Ė®µÄÖŲŅŖĶ¾¾¶Ö®Ņ»”£Ņ»°ćŹĒĻČ½«ŗ£Ė®µ»Æ»ńµĆµĖ®£¬ŌŁ“ÓŹ£ÓąµÄÅØŗ£Ė®ÖŠĶعżŅ»ĻµĮŠ¹¤ŅÕĮ÷³ĢĢįČ”ĘäĖū²śĘ·”£
»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)ĻĀĮŠøĽųŗĶÓÅ»Æŗ£Ė®×ŪŗĻĄūÓĆ¹¤ŅÕµÄÉčĻėŗĶ×ö·ØæÉŠŠµÄŹĒ________(ĢīŠņŗÅ)”£
¢ŁÓĆ»ģÄż·Ø»ńČ”µĖ®
¢ŚĢįøß²æ·Ö²śĘ·µÄÖŹĮæ
¢ŪÓÅ»ÆĢįČ”²śĘ·µÄĘ·ÖÖ
¢ÜøĽų¼Ų”¢äå”¢Ć¾µČµÄĢįČ”¹¤ŅÕ
(2)²ÉÓĆ”°æÕĘų“µ³ö·Ø”±“ÓÅØŗ£Ė®“µ³öBr2£¬²¢ÓĆ“æ¼īĪüŹÕ”£¼īĪüŹÕäåµÄÖ÷ŅŖ·“Ó¦ŹĒBr2£«Na2CO3£«H2O”śNaBr£«NaBrO3£«NaHCO3£¬ĪüŹÕ1 mol Br2Ź±£¬×ŖŅʵĵē×ÓŹżĪŖ________mol”£
(3)ŗ£Ė®ĢįĆ¾µÄŅ»¶Ī¹¤ŅÕĮ÷³ĢČēĻĀĶ¼£ŗ

ÅØŗ£Ė®µÄÖ÷ŅŖ³É·ÖČēĻĀ£ŗ
| Ąė×Ó | Na£« | Mg2£« | Cl£ | SO |
| ÅضČ/(g”¤L£1) | 63.7 | 28.8 | 144.6 | 46.4 |
øĆ¹¤ŅÕ¹ż³ĢÖŠ£¬ĶŃĮņ½×¶ĪÖ÷ŅŖ·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ______________________________£¬²śĘ·2µÄ»ÆѧŹ½ĪŖ__________£¬1 LÅØŗ£Ė®×ī¶ąæɵƵ½²śĘ·2µÄÖŹĮæĪŖ________g”£
(4)²ÉÓĆŹÆÄ«Ńō¼«”¢²»ŠāøÖŅõ¼«µē½āČŪČŚµÄĀČ»ÆĆ¾£¬·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ________________________£»µē½āŹ±£¬ČōÓŠÉŁĮæĖ®“ęŌŚ»įŌģ³É²śĘ·Ć¾µÄĻūŗÄ£¬Š“³öÓŠ¹Ų·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ____________________________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ijČÜŅŗÖŠÖ»æÉÄÜŗ¬ÓŠK+”¢NO3−”¢SO42−”¢NH4+”¢CO32−ÖŠµÄ¼øÖ֣ز»æ¼ĀĒÉŁĮæµÄH+ÓėOH−£©£¬Č”200 mLøĆČÜŅŗ·Ö³ÉĮ½µČ·Ż£¬½ųŠŠČēĻĀŹµŃé£ŗŅ»·Ż¼ÓČė×ćĮæÉÕ¼ī²¢¼ÓČČ£¬²śÉśĘųĢåŌŚ±ź×¼×“æöĻĀĪŖ224 mL£»ĮķŅ»·ŻĻČ¼Ó×ćĮæŃĪĖįĪŽĻÖĻó£¬ŌŁ¼ÓČė×ćĮæBaCl2µĆµ½2.33 g¹ĢĢ壬ŌņøĆČÜŅŗÖŠ ””
A£®æÉÄÜŗ¬ÓŠK+ B£®æĻ¶Øŗ¬ÓŠNO3−”¢SO42−”¢NH4+”¢CO32−
C£®Ņ»¶Ø²»ŗ¬ÓŠNO3− D£®Ņ»¶Øŗ¬ÓŠK+
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com