
���������ΰ�һ��������Ϻ��ۣ����Ƶû�����X��X����ˮ�ܵ����K����Cr3+��SO42��������2��83 g X�е�Cr3+ȫ������ΪCr2O72������Һ�е�Cr2O72���ɺ���KI��Һ��Ӧ���õ�3��81g I2����Ӧ�����ӷ���ʽΪ��Cr2O72����I����H����Cr3+��I2��H2O��δ��ƽ�� ����������2��83 g X����Һ�У����������BaCl2��Һ���ɵõ�4��66 g��ɫ�������ɴ˿��ƶϳ�X�Ļ�ѧʽΪ �� ��
A��![]() �� B��
�� B��![]()
C��![]() �� D��
�� D��![]()

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���㽭ʡ����10�½��Բ��Ի�ѧ�Ծ� ���ͣ�ѡ����
���������ΰ�һ��������Ϻ��ۣ����Ƶû�����X��X����ˮ�ܵ����K����Cr3+��SO42��������2��83 g X�е�Cr3+ȫ������ΪCr2O72������Һ�е�Cr2O72���ɺ���KI��Һ��Ӧ���õ�3��81g I2����Ӧ�����ӷ���ʽΪ��Cr2O72����I����H����Cr3+��I2��H2O��δ��ƽ�� ����������2��83 g X����Һ�У����������BaCl2��Һ���ɵõ�4��66 g��ɫ�������ɴ˿��ƶϳ�X�Ļ�ѧʽΪ �� ��
A��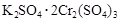 ��
B��
��
B��
C�� ��
D��
��
D��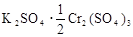
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꽭��ʡ������һ���¿���ѧ�Ծ� ���ͣ�ѡ����
�����������ΰ�һ��������Ϻ��ۣ����Ƶû�����X��X����ˮ�ܵ����K+��Cr3+��SO42-������2.83 g X�е�Cr3+ȫ������ΪCr2O72-����Һ�е�Cr2O72-�ɺ���KI��Һ��Ӧ���õ�3.81 g I2����Ӧ�����ӷ���ʽΪ��Cr2O72-+6I-+14H+=2Cr3++3I2+7H2O����������2.83 g X����Һ�У����������BaCl2��Һ���ɵõ�4.66 g��ɫ�������ɴ˿��ƶϳ�X�Ļ�ѧʽΪ�� ��
A��K2SO4��2Cr2(SO4)3 B��2K2SO4��Cr2(SO4)3
C��K2SO4��Cr2(SO4)3 D��K2SO4��1/2Cr2(SO4)3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�갲��ʡ�����и�����һ�ν�ѧ������⻯ѧ�Ծ� ���ͣ�ѡ����
���������ΰ�һ��������Ϻ��ۣ����Ƶû�����X��X����ˮ�ܵ����K+��Cr3+��SO2��4������2.83gX�е�Cr3+��ȫ������ΪCr2O2-7����Һ�е�Cr2O2-7�ɺ���KI��Һ��Ӧ���õ�3.81gI2����Ӧ�����ӷ���Ϊ��Cr2O2-7+6I��+14H+=2Cr3++3I2+7H2O����������2.83gX����Һ�У����������BaCl2��Һ���ɵõ�4.66g��ɫ�������ɴ˿��ƶϳ�X�Ļ�ѧʽΪ�� ��

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com