
H3PO2��NaH2PO2���ɽ���Һ�е�Ag����ԭΪ�����Ӷ������ڻ�ѧ������
��H3PO2�У�PԪ�صĻ��ϼ�Ϊ________��
������H3PO2���л�ѧ������Ӧ�У��������뻹ԭ�������ʵ���֮��Ϊ4��1������������Ϊ________(�ѧʽ)��
(3)H3PO2�Ĺ�ҵ�Ʒ��ǣ�������(P4)��Ba(OH)2��Һ��Ӧ����PH3�����Ba(H2PO2)2����������H2SO4��Ӧ��д��������Ba(OH)2��Һ��Ӧ�Ļ�ѧ����ʽ__________________________��
�𰸡�(2)�٣�1����H3PO4
(3)2P4��3Ba(OH)2��6H2O===3Ba(H2PO2)2��2PH3��
������(2)��H3PO2��PԪ�ػ��ϼ�Ϊ��1�ۡ�
�ڸ���H3PO2��Ag����Ӧ���������ʵ���֮��Ϊ1��4��4 mol Ag���ڷ�Ӧ�еõ�4 mol e����1 mol H3PO2��ʧȥ4 mol e��������PԪ�ؽ��ԣ�5�ۣ�����ΪH3PO4������������ΪH3PO4��
(3)����������ԭ��Ӧ����ʽ��ƽԭ��6H2O��2P4��3Ba(OH)2===2PH3����3Ba(H2PO2)2��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʵ���ҿ�����ͼ��ʾ��װ�ý���CO��CO2�������ķ�������֪aΪֹˮ�У�bΪ��Һ©�����Ļ������ɹ�ѡ�õ��Լ���NaHCO3��Һ��NaOH��Һ��NaHS��Һ��Ũ���ᡢ��ˮ�Ȼ��ơ�ϡ���ᡣ����ջش�

(1)���ƿ����Ӧ�ŵ��Լ�Ϊ________�����ƿ����Ӧ�ŵ��Լ�Ϊ________����Һ©������Ӧ�ŵ��Լ�Ϊ________��
(2)��һ��Ӧ�ȷ����________������ʱӦ�ȹر�________����________��������Ӧ�����ӷ���ʽΪ___________________________________________
__________________��
(3)�ڶ��������________ʱ���ȹر�________����________��������Ӧ�����ӷ���ʽΪ_____________________________________________________
___________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ǰ����(Li2NH)��һ�ִ��������ߡ���ȫ�ԺõĹ��崢����ϣ��䴢��ԭ���ɱ�ʾΪLi2NH��H2LiNH2��LiH�������й�˵����ȷ����(����)
A��Li2NH��N�Ļ��ϼ��ǣ�1
B���÷�Ӧ��H2�������������ǻ�ԭ��
C��Li����H�������Ӱ뾶���
D���˷������ƿ�����ԭ����ͬ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����FeS2��O2����Fe2O3��SO2���ش��������⣺
(1)������________����ԭ��________����������________����ԭ����________��
(2)Ԫ�ػ��ϼ����ߵ�Ԫ��Ϊ________��Ԫ�ػ��ϼ۽��͵�Ԫ��Ϊ________��
(3)1�����ӡ���ԭ�����ϼ���������Ϊ________��1�����ӡ����������ϼ۽�������Ϊ________��
(4)��ƽ������ʵ�ϵ������Ϊ____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ڷ�Ӧ3BrF3��5H2O===9HF��Br2��HBrO3��O2���У�����5 mol H2O�μӷ�Ӧ����ˮ��ԭ����Ԫ��Ϊ(����)
A��1 mol B.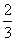 mol C.
mol C.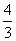 mol D��2 mol
mol D��2 mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��Ȼ���е�H2S���ʳ��ð�ˮ���գ�����ΪNH4HS��һ����������NH4HS��Һ��ͨ��������õ�������ʹ����Һ������д��������Ӧ�Ļ�ѧ����ʽ��________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
OH�����ܺ�________________________________________________________��������(��������ӣ���ͬ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij�����ĩ���п��ܺ���K2CO3��KNO3��NaNO2��K2SO3��Na2SO4��FeO��Fe2O3�е������֣�ijͬѧΪȷ���ù����ĩ�ijɷ֣�ȡ��������ʵ�飬ʵ����̼��������£�

��ͬѧ�ó��Ľ�����ȷ����(����)
A����������1���Ƴ��ù����ĩ�к�����Ԫ�أ���������Ԫ��
B����������2���Ƴ��ù����ĩ��һ������NaNO2
C����������3���Ƴ��ù����ĩ��һ������Na2SO4
D����������4������5���Ƴ��ù����ĩ��һ������FeO��Fe2O3
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com