
| T/K | T1 | 573 | T2 |
| K | 1.00×107 | 2.45×105 | 1.88×103 |
 ����ʱN2��ת����Ϊ ��
����ʱN2��ת����Ϊ �� 4NO+6H2O 4NO+3O2+2H2O=4HNO3
4NO+6H2O 4NO+3O2+2H2O=4HNO3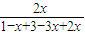 =
=
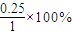 =25%��
=25%��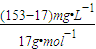 ×10-3g/mg×600m3×103L/m3=4800mol��
×10-3g/mg×600m3×103L/m3=4800mol�� 4NO+6H2O��4NO+3O2+2H2O=4HNO3
4NO+6H2O��4NO+3O2+2H2O=4HNO3

 ���Ǽ���С����ϵ�д�
���Ǽ���С����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| T/K | T1 | 573 | T2 |
| K | 1.00��107 | 2.45��105 | 1.88��103 |
| 1 |
| 7 |
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�����ɹŰ���һ�и�һ��ѧ����ĩ���Ի�ѧ�Ծ����������� ���ͣ������
��6�֣�����������ʳ��������������ũҵ�Ի�ѧ���ϵ�������Խ��Խ�����е���������������һ�ֻ��ʡ������ĺϳ�Ϊ���ʵ�������ҵ�춨�˻�������ԭ��Ϊ��N2��3H2 2NH3
2NH3
��1����N2��3H2 2NH3�ķ�Ӧ�У�һ��ʱ���NH3��Ũ��������0.9mol��L��1����N2��ʾ�䷴Ӧ����Ϊ0.15 mol��L��1��s��1������������ʱ��Ϊ ��
2NH3�ķ�Ӧ�У�һ��ʱ���NH3��Ũ��������0.9mol��L��1����N2��ʾ�䷴Ӧ����Ϊ0.15 mol��L��1��s��1������������ʱ��Ϊ ��
A��2 s B��3 s C��4 s D��6 s
��2������4���������ڲ�ͬ�����²�õĺϳɰ���Ӧ�����ʣ����з�Ӧ������ ��
A��v(H2)��0.1 mol��L��1��min��1 B��v(N2)��0.1 mol��L��1��min��1
C��v(NH3)��0.15 mol��L��1��min�� D��v(N2)��0.002mol��L��1��min��1
��3����һ�����ȡ��ݻ�������ܱ������з������淴Ӧ��
N2(g)��3H2(g) 2NH3(g) ��H��0�����и�����˵���÷�Ӧ�Ѿ��ﵽƽ��״̬���� ��
2NH3(g) ��H��0�����и�����˵���÷�Ӧ�Ѿ��ﵽƽ��״̬���� ��
A�������������ܶȱ��ֲ���
B���������¶Ȳ��ٱ仯
C������1mol N��N����ͬʱ������6 mol N��H��
D����Ӧ����N2��H2�����NH3������֮��1�U3�U2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| T/K | T1 | 573 | T2 |
| K | 1.00��107 | 2.45��105 | 1.88��103 |
| 1 |
| 7 |
| ||
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com