
X��Y��Z��W��ԭ��������������Ķ�����Ԫ�أ��һ���ͬ�壬����ֻ��һ��Ϊ������X�ĺ�������Ų�ʽΪnsnnpn��Z������������������Ӳ�����2���� Yԭ����Zԭ�ӵ�����������֮��Ϊ9����Y��W���ʶ������ռ���Һ��Ӧ����ش��������⣺
(1)Y��Z��W��ԭ�Ӱ뾶�ɴ�С��˳����________________(��Ԫ�ط���)
(2)XZ2�ĵ���ʽ��________________���ռ�ṹΪ________________���γɾ���ʱ����________________���塣
(3)Y�ļ۵����Ų�ʽ��________________����ҵ����������Y��ԭ����________________(�û�ѧ��Ӧ����ʽ��ʾ)
(4)������������ԭ�������ñ���ʳ��ˮ�ռ�W���ʵ�ԭ��________________��
(1)Al��S��Cl
(2)![]() ֱ���� ����
ֱ���� ����
(3)3s23p1
2Al2O3 ![]() 4Al+3O2��
4Al+3O2��
(4)Cl2+H2O ![]() H++Cl-+HClO������ʳ��ˮCl-Ũ�ȴ�ʹ����ƽ�������ƶ���������Cl2��ˮ�е��ܽ��
H++Cl-+HClO������ʳ��ˮCl-Ũ�ȴ�ʹ����ƽ�������ƶ���������Cl2��ˮ�е��ܽ��
������X��Y��Z��W��Ԫ�طֱ�ΪC��Al��S��Cl
(1)Al��S��Cl
(2)XZ2����ʽ��![]()
�ռ乹��Ϊֱ���Σ��������ڷ��Ӿ���
(3)Al�ļ۵����Ų�ʽΪ3s23p1����ҵ������ԭ��
2Al2O3 ![]() 4Al+3O2��
4Al+3O2��
(4)Cl2����ˮ������ƽ��Cl2+H2O ![]() H++Cl-+HClO������ʳ��ˮ�У�Cl-Ũ�Ƚϴ�ʹ����ƽ�������ƶ���������Cl2��ˮ�е��ܽ�ȡ�
H++Cl-+HClO������ʳ��ˮ�У�Cl-Ũ�Ƚϴ�ʹ����ƽ�������ƶ���������Cl2��ˮ�е��ܽ�ȡ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������и��⣺
(1)ǰ������Ԫ���У���̬ԭ����δ�ɶԵ���������������������ͬ��Ԫ����________�֡�
(2)�ڢ�A����A��Ԫ����ɵĻ�����GaN��GaP��GaAs�����˹��ϳɵ����Ͱ뵼����ϣ��侧��ṹ�뵥�������ơ�Gaԭ�ӵĵ����Ų�ʽΪ______________����GaN�����У�ÿ��Gaԭ����__________��Nԭ����������ͬһ��Gaԭ��������Nԭ�ӹ��ɵĿռ乹��Ϊ________�����Ĵ��������У�GaN����_______���塣
(3)�ڼ��Է���NCl3�У�Nԭ�ӵĻ��ϼ�Ϊ-3,Clԭ�ӵĻ��ϼ�Ϊ+1,���Ʋ�NCl3ˮ�����Ҫ������_________(�ѧʽ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ǵ����ϼ�Ϊ�ḻ��Ԫ�ء�
��1��Li3N�����е���N3-���ڣ���̬N3-�ĵ����Ų�ʽΪ ��
��2��N��N�ļ���Ϊ942 kJ��mol-1��N-N�����ļ���Ϊ247 kJ��mol-1������˵��N2�е� ���� ���ȶ����![]() ����
����![]() ������
������
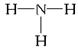
��3����CH3��3NH+��![]() ���γ�����Һ�塣����Һ����������������ɣ��۵����100�棬��ӷ���һ����л��ܼ� �����С������������ ������ţ���
���γ�����Һ�塣����Һ����������������ɣ��۵����100�棬��ӷ���һ����л��ܼ� �����С������������ ������ţ���
a. ��ȼ��
b.����ɫ���ܼ�
c. ���ϲ���
d. ���Ȳ���
��4��X+�����е������ó���K��L��M�������Ӳ㣬����N3-�γɵľ���ṹ��ͼ��ʾ��X��Ԫ�ط����� ����ͬһ��N3-������X+�� ����
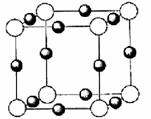
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
(A)�����ʽṹ�����ʡ�
�±���Ԫ�����ڱ���һ���֡��������е���ĸ�ֱ����ijһ�ֻ�ѧԪ�ء�

(1)T3+�ĺ�������Ų�ʽ��____________��
(2)Q��R��M�ĵ�һ�������ɴ�С��˳����___________________(��Ԫ�ط��ű�ʾ)��
(3)�����й�����Ԫ�ص�˵���У���ȷ����______________________(�����)��
��G���ʵ��۵����J���ʣ�����ΪG���ʵĽ�������ǿ
��J��X���ã�����J��������Һ���û���X
�۽�J
��RE3�е����QE4����Ҫ����Ϊǰ����Է��������ϴ�
��һ��Q2E4�����к�������Ҽ���һ���м�
(4)���ô�����̨��̫�շ�����EQ9R����֪����������ԭ�Ӿ��γ�8���ӻ�2�����ȶ��ṹ����ֱ���η��ӣ���������λ����д����ṹʽ��_________________��
(5)G��R����ֱ�ӻ�������һ�����ӻ�����G3R���þ����������ʯī�IJ�״�ṹ��ÿ���У�Gԭ�ӹ���ƽ�������Σ�ÿ�������ε�������һ��Rԭ�ӡ������֮�仹����һ��������ԭ�ӡ�������Щ���ӵ�ԭ��Ӧ����____________(��G��R��Ԫ�ط���)��

(B)��ʵ�黯ѧ��
ij������ʾ����ʹ˫��ˮ�ֽ�Ĵ����кܶ��֣��������(������)�������ʹ���(��FeCl3)�������(��MnO2)�ȶ��ǽϺõĴ�����ijʵ��С��ͨ���ⶨ˫��ˮ�ֽ������O2��ѹǿ��̽���ֽ�����������Ѵ����Լ�̽����Ѵ������ʵĴ�������
(һ)̽��һ��
ʵ�鲽��
(1)����ƿ�м���50 mL 1.5����˫��ˮ
(2)�ֱ�����ƿ�м�
(3)�ɼ��ͼ�¼���ݡ�
(4)�������ݵó��±�
��ͬ������ѹǿ��ʱ��б�ʡ��ıȽ�
���� | ���� | ������ | �Ȼ�ͭ | �Ȼ��� | ����ͭ | �������� |
ѹǿ��ʱ���б�� | 0.191 87 | 0.002 42 | 0.007 93 | 0.030 5 | 0.015 47 | 1.833 6 |
�ٸá�̽��һ��ʵ���������_____________________________________________________��
�ڸ�ʵ�����ó��Ľ�����_______________________________________________________��
(��)̽�������������̴�����Ѵ�����
��ʵ��С���ͬѧ�ڽ���̽������ʵ��ʱ���õ���һϵ�е�ͼ�������ݡ��ο���ͼ�ͱ���ֱ�ش�������⡣

3%��˫��ˮ�벻ͬ�����������̵�ѹ����ʱ��ͼ
������ͬŨ�ȵ�˫��ˮ�ڲ�ͬ�����Ķ��������������ռ���ͬ״����ͬ���O2����ʱ��
MnO2 ʱ�� H2O2 | |||
1.5�� | 223 s | 67 s | 56 s |
3.0�� | 308 s | 109 s | 98 s |
4.5�� | 395 s | 149 s | 116 s |
����ͼ�������������ǿ��Եó���
��ͬŨ�ȵ�˫��ˮ�ķֽ��������Ŷ����������������Ӷ�_________________�������Ӧʱ��_______________��
�������ʵ�����ͽ�ʡҩƷ�ĽǶ��ۺϷ���������Ϊ������ѡ��3.0%��˫��ˮ������___________ g�Ķ���������ʹʵ��Ч����ѡ����жϵ�������______________________��
�ݸ�С���ijͬѧͨ���������ݵó��˵�����������ͬʱ˫��ˮ��Ũ��ԽС��Ӧ����Խ��Ľ��ۣ�����Ϊ�Ƿ���ȷ____________�����������________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ԭ���������������A��B��C��D��E��F(A��B��C��D��E��F�ֱ����Ԫ�ط���)���ֶ�����Ԫ�ء�Ԫ��A��ԭ�Ӱ뾶�ڶ���������С��Ԫ��C�ĵ����ڿ����к�����࣬D+��F-��һ�����Ӳ㣬E�������ڵĸ�Ԫ�ص��ʷе�仯����ͼ(Ԫ�ذ�ԭ����������˳����������)��B��C����Ԫ�طֱ�����A�γɵȵ��ӵļס������ַ��ӣ������ַ����и�ԭ�ӵĸ������±���
������ | �� | �� |
ԭ�Ӹ����� | B��A=1��4 | C��A=1��3 |
(1)Ԫ��E�Ļ�̬ԭ�ӵĵ����Ų�ʽΪ________________________��
(2)д���������ҵĽṹʽ__________________���÷�����C��ԭ�ӹ����������____________�ӻ���
(3)B��C�γɵ�һ�ֻ�����X��һ��ԭ�Ӿ��壬������B��Cԭ�Ӿ��ﵽ8�����ȶ��ṹ����X�Ļ�ѧʽΪ__________________��
(4)D��F��ȼ�յIJ�������______________���壬�侧������D+����ҵȾ����F-��___________����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ����C7H8Ϊԭ�Ϻϳ�ij������������(C7H5NO)n������ͼ��

��֪��(��)���������Ѿ�����һ��ȡ����ʱ����������ȡ��������ԭȡ������Ӱ���ȡ�����ڡ���λ���λ����ԭ�ӡ�ʹ��ȡ�������������ڡ���λ��ȡ�����С�CH3����NH2�ȣ�ʹ��ȡ�����������ļ�λ��ȡ�����С�COOH����NO2�ȣ�
(��)R��CH=CH��R��![]() R��CHO+R�䡪CHO
R��CHO+R�䡪CHO
(��)����(��NH2)�ױ�����������(��NO2)�ɱ�Fe�����ỹԭ�ɰ���(��NH2)
�ش��������⣺
(1)X��Y��������ѡ���е�ijһ�����X��_________________��(��д���)
A.Fe������
B.����KMnO4��Һ
C.NaOH��Һ
(2)��֪B��F��Ϊͬ���칹�壬д������F�Ľṹ��ʽ__________________��
(3)�ֱ�д��D��E�еĺ��������ŵ�����________________��________________��
(4)������C������˵���У���ȷ����_______(��д���)��
A.�ܷ���������Ӧ
B.������ˮ�����ӳɷ�Ӧ
C.ֻ�ܺͼӦ���ܺ��ᷴӦ
(5)д����Ӧ�ߵĻ�ѧ����ʽ����ע����Ӧ���͡�
____________________________________________________________________��
____________________________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com