
C6H8O6+I2![]() C6H6O6+2HI
C6H6O6+2HI
�����ⶨij��Ʒ��ά����C�ĺ���������IJ��輰��õ��������¡�ȡ10 mL 6 mol��L-1CH3COOH������100 mL����ˮ������Һ������к������ȴ����ȷ��ȡ0.200 0 g��Ʒ���ܽ���������ȴ����Һ�С�����1 mL����ָʾ����������Ũ��Ϊ0.050 00 mol��L-1��I2��Һ���еζ���ֱ����Һ�е���ɫ��������Ϊֹ��������21.00 mL I2��Һ��
��1��Ϊ�μ����CH3COOHϡ��ҺҪ�Ⱦ���У���ȴ�����ʹ�ã�
��2��������Ʒ��ά����C������������

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
C6H8O6+I2![]() C6H6O6+2HI?
C6H6O6+2HI?
�����ⶨij��Ʒ��ά����C�ĺ���������IJ��輰��õ��������¡�ȡ10 mL 6 mol��L-1CH3COOH������100 mL����ˮ������Һ������к������ȴ����ȷ��ȡ0.200 0 g��Ʒ���ܽ���������ȴ����Һ�У�����1 mL����ָʾ����������Ũ��Ϊ0.050 00 mol��L-1��I2��Һ���еζ���ֱ����Һ�е���ɫ��������Ϊֹ��������21.00 mL I2��Һ��?
��1��Ϊ�μ����CH3COOHϡ��ҺҪ�Ⱦ���С���ȴ�����ʹ�ã�?
��2��������Ʒ��ά����C������������?
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�긣��ʡ�Ϻ�һ�и߶���ѧ����ĩ���Ի�ѧ�Ծ����������� ���ͣ�ʵ����
��6�֣�ÿ�ո�2�֣�ά����C����������Ѫ�ᣬ����ʽΪC6H8O6�����н�ǿ�Ļ�ԭ�ԣ������ڿ������ױ��������京����ͨ��������������Һ����I2��Һ���еζ���
�÷�Ӧ�Ļ�ѧ����ʽΪ��C6H8O6��I2��C6H6O6��2HI
�����ⶨij��Ʒ��ά����C�ĺ���������IJ��輰��õ��������£�
��ȡ10mL6mol��L��1CH3COOH������100mL����ˮ������Һ������к������ȴ��
��ȷ��ȡ0.2000g��Ʒ���ܽ���������ȴ����Һ�У�������1mL����ָʾ����
�����������Ũ��Ϊ0.0500 mol��L��1��I2��Һ���еζ���ֱ����Һ�е���ɫ��������Ϊֹ��������21.00mLI2��Һ��
��1��Ϊ�μ����CH3COOHϡ��ҺҪ�Ⱦ���С���ȴ�����ʹ��?
����е�ԭ��
����ȴ�����ʹ�õ�ԭ��
��2���������㣬��Ʒ��ά����C�İٷֺ���Ϊ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ2010-2011ѧ�������ѧһ�ֹ��ز��ԣ�9�� ���ͣ�������
��10�֣�ά����C����������Ѫ�ᣬ����ʽΪC6H8O6�����н�ǿ�Ļ�ԭ�ԣ������ڿ������ױ��������京����ͨ������������Һ������֪Ũ�ȵ�I2��Һ���вⶨ���÷�Ӧ�Ļ�ѧ����ʽ���£�C6H8O6��I2 C6H6O6��2HI�������ⶨij��Ʒ��ά����C�ĺ���������IJ��輰��õ��������¡�ȡ10 mL 6 mol/L CH3COOH������100 mL����ˮ������Һ������к������ȴ����ȷ��ȡ0.2000 g��Ʒ���ܽ���������ȴ����Һ�С�����1 mL����ָʾ����������Ũ��Ϊ0.05000 mol/L��I2��Һ���еζ���ֱ����Һ�е���ɫ��������Ϊֹ��������21.00 mL I2��Һ��
��1��Ϊ�μ����CH3COOHϡ��ҺҪ�Ⱦ���С���ȴ�����ʹ�ã�
��2��������Ʒ��ά����C������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�����ʡ������ѧ�ڵ������¿���ѧ�Ծ� ���ͣ�ѡ����
ά����C(VitaminC)��������Ѫ�ᣬ�������Ժ�ǿ��ԭ�ԣ�Ҳ��һ�ֳ�����ʳƷ���Ӽ�����ṹ����ͼ��
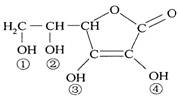
�����й�˵������ȷ����
A��ά����C�ķ���ʽΪC6H10O6
B��ά����C���ں���������������ˮ
C��ά����C���ں���C=O�����ܷ���������Ӧ
D��ά����C�����Կ����Ǣۡ��������ǻ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��㶫ʵ����ѧ�߶���ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ�ѡ����
ά����C(VitaminC)��������Ѫ�ᣬ�������Ժ�ǿ��ԭ�ԣ�Ҳ��һ�ֳ�����ʳƷ���Ӽ�����ṹ����ͼ�������й�˵������ȷ����

A��ά����C�ķ���ʽΪC6H10O6
B�����õ�ά����C���ױ���������������
C��ά����C���ں���C=O�����ܷ���������Ӧ
D��ά����C�����Կ����Ǣۡ��������ǻ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com