
��16�֣�����β���dz��п�������Ҫ��Ⱦ��֮һ������Ҫ�к��ɷ���CO���������NOx���ȡ�
��1��NOx������ԭ��֮һ����������������ʱ����N2��O2��Ӧ���������仯ֵ����ͼ��ʾ��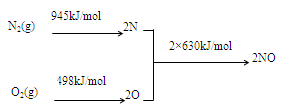
��N2(g)��O2(g) 2NO(g) ��H=���� ��
2NO(g) ��H=���� ��
��2������β����CO��NOx����Ч������Ϊ�����������Ҫ���⡣ij�о�С����ʵ������ij���ʹ�����CO��NO��ת�������о������NOת��ΪN2��ת�������¶ȡ�CO������ı仯�������ͼһ��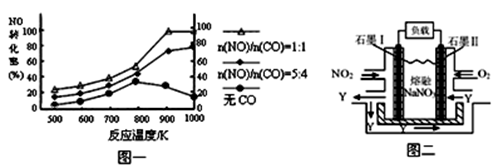
�� NO��CO���ʱ�����Ӧ�Ļ�ѧ����ʽΪ ��
�� 1000K��n(NO)/n(CO)=5:4ʱ��NO��ת����Ϊ75%����CO��ת����ԼΪ ��
�� ����n(NO)/n(CO)��ʵ�ʹ������Dz��ϱ仯�ģ���֤NOת���ʽϸߵĴ�ʩ�ǽ��¶ȴ�Լ������ K֮�䡣
��3������β����NOx����ͨ��ȼ�ϵ��ʵ��ת�����Ѿ������� NO2��O2������NaNO3�Ƴ���ȼ�ϵ�أ���ԭ����ͼ����
�� ͼ��ʯī��Ϊ��ص� ����
�� �ڸõ��ʹ�ù����У�ʯīI�缫�ϵIJ�����������Y����缫��ӦʽΪ ��
��4���״�Ҳ������ȼ�ϵ�ء���ҵ�ϲ��÷�ӦCO2(g)+3H2(g)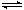 CH3OH(g)+H2O(g) ��H<0�ϳɼ״���
CH3OH(g)+H2O(g) ��H<0�ϳɼ״���
�� �ں����ܱշ�Ӧ���У�H2��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�
ϵ��ͼ����ʾ����A��B��C���㴦��Ӧƽ�ⳣ��(KA��KB��KC)��
��С��ϵΪ ��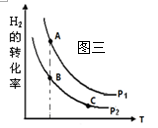
�� ij�����£���6molCO2��8molH2����2L�ܱ������з���
��Ӧ���ﵽƽ�����c(CO2)=2.0mol��L-1������¶��·�Ӧ��ƽ
�ⳣ��ֵΪ ��
(16�֣�ÿ��2��)
��1�� +183 kJ��mol��1��2�֣�δд��λ��δд��+������1�֣�
��2����2CO+2NO��N2 +2CO2��2�֣�δ��ƽ��1�֣��� ��93%����NOת������ý��Ϊ93.75%��������NO�����ֽ��Ӱ�죬ʵ��ֵ�������ֵ�в�࣬���Իش�93.75%�������ֵ�����֣� ��800��900
��3���� NO2��NO3�� ��e�� ��N2O5
��4����KA��KB>KC ��1/2
���������������1����ͼ��֪���������ӵĹ��ۼ�������Ҫ����945kJ���������������ӵĹ��ۼ�������Ҫ����498kJ����������2N��2O��ϳ�2��NO���ӷų�1260kJ�����������ԡ�H=945+498-1260=+183kJ/mol��
��2����NO��CO���ʱ�����ɵ����Ͷ�����̼����ѧ����ʽΪ2CO+2NO��N2 +2CO2��
��1000K��n(NO)/n(CO)=5:4ʱ��NO��ת����Ϊ75%����NO�����ʵ�����5mol����CO�����ʵ�����4mol,����NO�����ʵ�����5mol��75%=3.75mol��������CO �����ʵ�����3.75mol������CO��ת����Ϊ3.75mol/4mol��100%=93.75%��
����ͼһ��֪����n(NO)/n(CO)Ϊ��ֵ��800-900KʱNO��ת���ʽϸߣ�����900K��������CO����ʱ��NO��ת�����������ߣ�����COʱNO��ת����ȴ���½������������˵��¶���800-900K֮�䣻
��3������������ԭ��Ӧ������ͨ�������ļ��ǵ�ص�������ʯīIͨ����Ƕ����������壬����������Ӧ������������NԪ�صĻ��ϼ���+4�ۣ�NԪ�ػ��ϼ�����ֻ�����ߵ�+5�ۣ�����������Y��N2O5���缫��ӦʽΪNO2��NO3�� ��e�� ��N2O5
��4����A��B����¶���ͬ������ƽ�ⳣ����ͬ��C���¶ȸ���B���¶����ߣ�������Ӧ������ƽ�������С���������ߵĴ�С��ϵ��KA��KB>KC��
��6molCO2��8molH2����2L�ܱ������з���CO2(g)+3H2(g) CH3OH(g)+H2O(g) ƽ�����c(CO2)=2.0mol��L-1�������Ķ�����̼��Ũ����1mol/L,����������Ũ����3mol/L�����ɵļ״���ˮ������Ũ�ȶ���1mol/L,������ƽ��Ũ����mol/L,����K=c(CH3OH)c(H2O)/c(CO2)c(H2)3=1/2
CH3OH(g)+H2O(g) ƽ�����c(CO2)=2.0mol��L-1�������Ķ�����̼��Ũ����1mol/L,����������Ũ����3mol/L�����ɵļ״���ˮ������Ũ�ȶ���1mol/L,������ƽ��Ũ����mol/L,����K=c(CH3OH)c(H2O)/c(CO2)c(H2)3=1/2
���㣺���鷴Ӧ�ȵļ��㣬��ѧƽ�ⳣ�����жϼ����㣬�绯ѧ���۵�Ӧ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(8��)��1���£�N2H4���ֳ���������һ�ֿ�ȼ�Ե�Һ�壬���������ȼ�ϡ���֪��101kPaʱ��32��0gN2H4����������ȫȼ�����ɵ������ų�����624kJ��25��ʱ����N2H4��ȼ���Ȼ�ѧ����ʽ�� ��
��2���¡�����ȼ�ϵ����һ�ּ���ȼ�ϵ�أ��������Һ��20%~30%��KOH��Һ��
�¡�����ȼ�ϵ�طŵ�ʱ�������ĵ缫��Ӧʽ�� ��
��3����ƿ�����õ��һ��ΪǦ���أ�����һ�ֵ��͵Ŀɳ���أ�����ܷ�ӦʽΪ��Pb+PbO2+4H++2SO42- 2PbSO4+2H2O�����ʱ��������Ӧʽ�� �ô�װ�õ��ˮ����ˮ��D2O����ɵĻ��Һ�����缫����Pt����ͨ��һ��ʱ������������ռ���33��6 L����״�������壬������Ϊ18.5 g������������Hԭ�Ӻ�Dԭ�Ӹ���֮�ȣ�
2PbSO4+2H2O�����ʱ��������Ӧʽ�� �ô�װ�õ��ˮ����ˮ��D2O����ɵĻ��Һ�����缫����Pt����ͨ��һ��ʱ������������ռ���33��6 L����״�������壬������Ϊ18.5 g������������Hԭ�Ӻ�Dԭ�Ӹ���֮�ȣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1���ϳɰ���ҵ������������Ȼ����ˮ��Ӧ�Ʊ�������Ҫ��ӦΪ��
CH4(g)+ 2H2O(g)  CO2(g)+4H2(g)
CO2(g)+4H2(g)
��Ӧ�����������仯��ͼ��ʾ��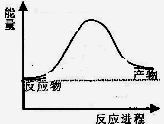
��÷�ӦΪ ��Ӧ������ȡ����ȡ���
����֪���ƻ�1mol��ѧ����Ҫ���յ��������±���ʾ��
| ��ѧ�� | C��H | O��H | C=O | H��H |
| ����������kJ/mol�� | a | b | c | d |
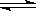 CO2(g)+4H2 (g)��Ӧ�� ��Ӧ���е�4sʱCO2��Ũ��Ϊ0.08mol/L���ٹ�һ��ʱ�䷴Ӧ��ƽ�⣬ƽ��ʱ������ѹǿ����ʼʱ��1.4����
CO2(g)+4H2 (g)��Ӧ�� ��Ӧ���е�4sʱCO2��Ũ��Ϊ0.08mol/L���ٹ�һ��ʱ�䷴Ӧ��ƽ�⣬ƽ��ʱ������ѹǿ����ʼʱ��1.4�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
CO�dz����Ļ�ѧ���ʣ��ڹ�ҵ��������;�ܹ㷺��
��1�� ��֪��ijЩ��Ӧ���Ȼ�ѧ����ʽ���£�
2H2��g��+SO2��g��=S��g��+2H2O��g�� ��H��+90.4kJ��mol��1
2CO��g��+O2��g��=2CO2��g�� ��H��-556.0kJ��mol��1
2H2��g��+O2��g��=2H2O��g�� ��H��-483.6kJ��mol��1
��д����CO��ȥ������SO2������S��g����CO2�Ȼ�ѧ����ʽ
��2�� ijȼ�ϵ����COΪȼ�ϣ��Կ���Ϊ��������������̬��K2CO3Ϊ����ʣ���д����ȼ�ϵ�������ĵ缫��Ӧʽ ��
��3����ij�¶��¡��ݻ���Ϊ2L�������ܱ������У�����ͬ��ʽͶ�뷴Ӧ����ֺ��º��ݣ�ʹ֮������Ӧ��2H2��g����CO��g�� CH3OH��g������H����dJ��mol��1��d��0������ʼͶ����������ﵽƽ��ʱ���й��������£�
CH3OH��g������H����dJ��mol��1��d��0������ʼͶ����������ﵽƽ��ʱ���й��������£�
| ʵ�� | �� | �� | �� |
| ��ʼͶ�� | 2 molH2��1 molCO | 1 mol CH3OH | 4 molH2��2 molCO |
| ƽ��ʱn��CH3OH �� | 0.5mol | n2 | n3 |
| ��Ӧ�������仯 | �ų�Q1kJ | ����Q2kJ | �ų�Q3kJ |
| ��ϵ��ѹǿ | P1 | P2 | P3 |
| ��Ӧ���ת���� | ��1 | ��2 | ��3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��14�֣����û�ѧ��Ӧԭ�����������������
��1����֪�� ��CO(g)+2H2(g)  CH3OH(g) ��Hl= ��91kJ��mol��l
CH3OH(g) ��Hl= ��91kJ��mol��l
��2CH3OH(g) CH3OCH3(g)+H2O(g) ��H2= ��24 kJ��mol��l ��CO(g) +H2O(g)
CH3OCH3(g)+H2O(g) ��H2= ��24 kJ��mol��l ��CO(g) +H2O(g)  CO2(g)+H2(g) ��H3= ��41 kJ��mol��l
CO2(g)+H2(g) ��H3= ��41 kJ��mol��l
��������Ӧ��ƽ�ⳣ������ΪK1��K2��K3 ��Ӧ 3CO(g) +3H2(g) CH3OCH3(g) +CO2(g) ��H= .
��Ӧ 3CO(g) +3H2(g) CH3OCH3(g) +CO2(g) ��H= .
��ѧƽ�ⳣ��K= ���ú�K1��K2��K3�Ĵ���ʽ��ʾ����
��2��һ�������£����������Ϊ1:2��CO��H2����ͨ�����һ�����ܱ������з�����Ӧ 3CO(g) +3H2(g) CH3OCH3(g) +CO2(g)��������˵����Ӧ�ﵽƽ��״̬�� ��
3CO(g) +3H2(g) CH3OCH3(g) +CO2(g)��������˵����Ӧ�ﵽƽ��״̬�� ��
a����ϵѹǿ���ֲ��� B����������ܶȱ��ֲ���
c�� CO��H2�����ʵ������ֲ��� d��CO�������ٶȵ���CO2����������
��3����������ˮ�õ���ˮ����25���£���x mol��L��l�İ�ˮ��y mol��L��1������������ϣ���Ӧ����Һ�����ԣ���c(NH4+)____c��Cl�������>������<������=�������ú�x��y�Ĵ���ʽ��ʾ����ˮ�ĵ���ƽ�ⳣ�� .
��4����ѧ�ҷ�����ʹNH3ֱ������ȼ�ϵ�صķ�������װ���ò������缫������������Һ�У�һ���缫ͨ���������һ�缫ͨ��NH3�����ط�ӦʽΪ��4NH3+3O2 = 2N2+6H2O���������ҺӦ�� ������ԡ��������ԡ��������ԡ�����
д�������ĵ缫��Ӧ����ʽ .
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��14�֣�
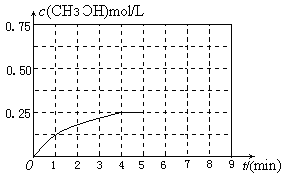
��.�ȼҵ�г������ӽ���Ĥ������Ƽ��ͼ1��ʾ����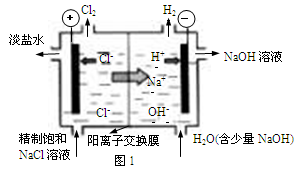
����1��д��ͼ1�������ĵ缫��Ӧʽ ��
����2����֪�����ӽ���Ĥֻ����������ͨ���������ӽ���Ĥֻ����������ͨ������ҵ������ͼ2װ�õ�ⱥ��Na2SO4��Һ������������NaOH��H2SO4�����װ������Ҫ��ȱ���� ��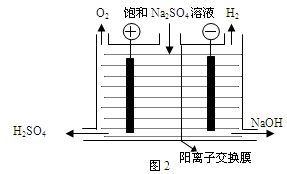
��.�����(MoS2)��һ����Ҫ�Ŀ��ͼ3�ǻ�����㱺��¯��ʾ��ͼ������1��2��3������¯���š�580��600��610�����Ǹ�¯����¶ȣ��棩��ͼ4�����˸�¯��������ϵ����ʵ����ٷֺ�����
��֪��MoS2��������1molMoO3�ķ�Ӧ�ȡ�H1=-1011KJ/mol��MoO2��������1molMoO3�ķ�Ӧ�ȡ�H2=-154KJ/mol���Իش�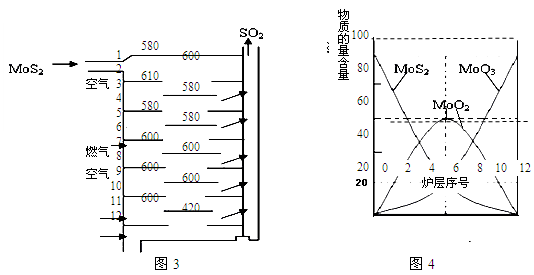
��1����֤����������ɵ�������SO2������SO3�ķ����� ��
��2������������ɵ�������ʹ�����ữ��KMnO4��Һ��ɫ���û�ѧ����ʽ��ʾ��ɫ��ԭ�� ��
��3����6¯����ڵĹ������ʷֱ���MoS2��MoO3��MoO2�������ǵ����ʵ���֮��Ϊ ��
��4��ͼ4�������м�¯�㣨4��6�����ܴ���һ�֡�����+���������+�����ķ�Ӧ����д���÷�Ӧ���Ȼ�ѧ��Ӧ����ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪�� C(s)��O2(g)��CO2(g) ��H����393��5 kJ/mol
2CO(g)��O2(g)��2CO2(g) ��H����566 kJ/mol
TiO2(s)��2Cl2(g)��TiCl4(s)��O2(g) ��H��+141 kJ/mol
��TiO2(s)��2Cl2(g)��2C(s)��TiCl4(s)��2CO(g) ��H�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�о������ḻ��CO2��ȫ������Ϊ��̼Դ�������ǰӦ����㷺��̼Դ(ʯ�ͺ���Ȼ��)����������Ҷ���ݽߵ�Σ����ͬʱ�ֿɻ�����CO2�ۻ�������������ЧӦ��ʵ��CO2������ѭ����
��1��Ŀǰ��ҵ����һ�ַ�������CO2��H2��230�����������ת�����ɼ״�������ˮ��������ͼ��ʾ��ѹ������0.5 mol CO2��1.5 mol H2ת���ʴ�80%ʱ�������仯ʾ��ͼ��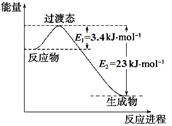
��д���÷�Ӧ���Ȼ�ѧ����ʽ�� ��
�����жϸ÷�Ӧ�ﵽ��ѧƽ��״̬�������� ��
a��������ѹǿ���� b��H2�������������
c��c(H2)=3c(CH3OH) d���������ܶȲ���
e��2��C��O���ѵ�ͬʱ��6��H��H���ѡ�
��2���˹���������ܹ�����̫���ܣ���CO2��H2O�Ʊ���ѧԭ�ϣ���ͼ��ͨ���˹���������Ʊ�HCOOHԭ����ʾ��ͼ������Ҫ��ش����⣺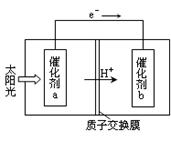
�ٸù����ǽ� ת��Ϊ ��(��������ѡ����ܡ���̫���ܡ�����ѧ�ܡ�)
�ڴ���b����ĵ缫��Ӧ����ʽΪ ��
��3��ij��������Ա�����ʹ�����������ͣ����ͻ�ѧʽ��C8H18��ʾ�����ȼ�ϵķ������Խ������CO2���ŷ����⡣�÷�����Ҫ���ô������CaH2����H2������������������������ʹ�ã�����ϵͳ�ֲ�������ȼ�ղ�����CO2����ϵͳ��Ӧ����ͼ��ʾ��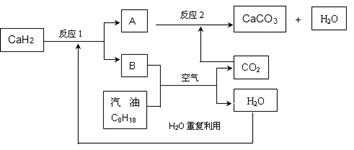
����������⣺
��д��CaH2�ĵ���ʽ ��
�ڷ�Ӧ1���������뻹ԭ�������ʵ���֮���ǣ� ��
�����ϵͳ��Ӧ��������ȫ����д����ϵͳ�ܷ�Ӧ�Ļ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com