
œ¬±μ÷–Ε‘άκΉ”ΖΫ≥Χ ΫΒΡΤάΦέ≤ΜΚœάμΒΡ «Θ®ΓΓΓΓΘ©
|



| ΡξΦΕ | ΗΏ÷–ΩΈ≥Χ | ΡξΦΕ | ≥θ÷–ΩΈ≥Χ |
| ΗΏ“Μ | ΗΏ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ“Μ | ≥θ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏΕΰ | ΗΏΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θΕΰ | ≥θΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏ»ΐ | ΗΏ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ»ΐ | ≥θ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ2013-2014―ßΡξ’ψΫ≠ ΓΗΏ»ΐ…œ―ßΤΎΤΎΡ©άμΉέΜ·―ß ‘ΨμΘ®ΫβΈωΑφΘ© Χβ–ΆΘΚ―Γ‘ώΧβ
œ¬±μ÷–Ε‘άκΉ”ΖΫ≥Χ ΫΒΡΤάΦέ≤ΜΚœάμΒΡ «
―Γœν????????????? Μ·―ßΖ¥”ΠΦΑάκΉ”ΖΫ≥Χ Ϋ????????????? ΤάΦέ
A????????????? NaClO»ή“Κ÷–Ά®»κ…ΌΝΩΒΡSO2ΘΚClOΘ≠ΘΪH2OΘΪSO2===ClΘ≠ΘΪSO42-ΘΪ2HΘΪ????????????? ¥μΈσΘ§Φν–‘Ϋι÷ ÷–≤ΜΩ…Ρή…ζ≥…HΘΪ
B????????????? ”ΟΥα–‘ΗΏΟΧΥαΦΊ»ή“ΚΒΈΕ®≤ίΥαΘΚ2MnO4-ΘΪ5H2C2O4ΘΪ6HΘΪ===2Mn2ΘΪΘΪ10CO2ΓϋΘΪ8H2O????????????? ’ΐ»Ζ
C????????????? NH4Al(SO4)2»ή“Κ÷–ΒΈ»κΦΗΒΈNaOH»ή“ΚΘΚNH4+ΘΪOHΘ≠===NH3ΓΛH2O????????????? ¥μΈσΘ§OHΘ≠ Ήœ»ΚΆAl3ΘΪΖ¥”Π…ζ≥…Al(OH)3≥ΝΒμ
D????????????? ”ΟΕη–‘ΒγΦΪΒγΫβMgCl2»ή“ΚΘΚ2Mg2ΘΪΘΪ2H2O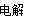 2MgΘΪO2ΓϋΘΪ4HΘΪ????????????? ’ΐ»Ζ
2MgΘΪO2ΓϋΘΪ4HΘΪ????????????? ’ΐ»Ζ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ2011-2012ΡξΚτ¬Ή±¥Εϊ –ΗΏΕΰœ¬―ßΤΎΤΎΡ©ΩΦ ‘Μ·―ß ‘ΨμΘ®ΫβΈωΑφΘ© Χβ–ΆΘΚ―Γ‘ώΧβ
œ¬±μ÷–Ε‘άκΉ”ΖΫ≥Χ ΫΒΡΤάΦέΚœάμΒΡ «
|
―Γœν |
Μ·―ßΖ¥”ΠΦΑάκΉ”ΖΫ≥Χ Ϋ |
ΤάΦέ |
|
A |
NaClO»ή“Κ÷–Ά®»κ…ΌΝΩΒΡSO2ΘΚ ClO-+H2O+SO2=Cl-+SO42-+2H+ |
¥μΈσΘ§Φν–‘Ϋι÷ ÷–≤ΜΩ…Ρή…ζ≥…H+ |
|
B |
Υα–‘ΗΏΟΧΥαΦΊ»ή“ΚΚΆH2O2÷Τ»Γ…ΌΝΩO2ΘΚ 4MnO4-+4H2O2+12H+=4Mn2++7O2Γϋ+10H2O |
’ΐ»Ζ |
|
C |
NH4Al(SO4)2»ή“Κ÷–ΒΈ»κΦΗΒΈNaOH»ή“ΚΘΚ NH4++OH-=NH3ΓΛH2O |
¥μΈσΘ§≤ζΈο÷–ΜΙ”–…ΌΝΩAl(OH)3…ζ≥… |
|
D |
”ΟΕη–‘ΒγΦΪΒγΫβMgCl2»ή“ΚΘΚ 2Mg2++2H2O |
’ΐ»Ζ |
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ2013ΫλΥΡ¥® ΓΗΏΕΰœ¬―ßΤΎ2‘¬―ß“ΒΦλ≤βΜ·―ß ‘Ψμ Χβ–ΆΘΚ―Γ‘ώΧβ
œ¬±μ÷–Ε‘άκΉ”ΖΫ≥Χ ΫΒΡΤάΦέ≤ΜΚœάμΒΡ «( )
|
―Γœν |
Μ·―ßΖ¥”ΠΦΑΤδάκΉ”ΖΫ≥Χ Ϋ |
ΤάΦέ |
|
AΘ° |
œρ¬»Μ·¬Ν»ή“Κ÷–Φ”»κΙΐΝΩ«β―θΜ·ΡΤ»ή“ΚΘΚ Al3+ + 4OH- = AlO2- + 2H2O |
’ΐ»Ζ |
|
BΘ° |
œρΧΦΥαΗΤ…œΒΈΦ”œΓ―ΈΥαΘΚ 2H+ + CO32- ΘΫ CO2Γϋ+ H2O |
¥μΈσΘ§ΧΦΥαΗΤ≤Μ”ΠΗΟ–¥≥…CO32- –Έ Ϋ |
|
CΘ° |
œρΝρΥαοß»ή“Κ÷–Φ”»κ«β―θΜ·±Β»ή“ΚΘΚ Ba2+ + SO42- == BaSO4Γΐ |
’ΐ»Ζ |
|
DΘ° |
ΧζΖέ”κœΓΝρΥαΖ¥”ΠΘΚ 2Fe+ 6H+ == 2Fe3+ + 3H2Γϋ |
¥μΈσΘ§ H+ΒΡ―θΜ·–‘Ϋœ»θΘ§÷ΜΡήΫΪΧζΒΞ÷ ―θΜ·ΈΣFe2+ |
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΑΌΕ»÷¬–≈ - ΝΖœΑ≤αΝ–±μ - ‘ΧβΝ–±μ
Κΰ±± ΓΜΞΝΣΆχΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΤΫΧ® | Άχ…œ”–ΚΠ–≈œΔΨΌ±®Ή®«χ | Βγ–≈’©Τ≠ΨΌ±®Ή®«χ | …φάζ Ζ–ιΈό÷ς“ε”–ΚΠ–≈œΔΨΌ±®Ή®«χ | …φΤσ«÷»®ΨΌ±®Ή®«χ
ΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΒγΜΑΘΚ027-86699610 ΨΌ±®” œδΘΚ58377363@163.com