
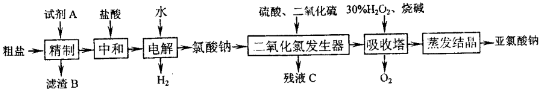
£Ø2011?Äž²ØÄ£Äā£©”°»ÆѧÓė¼¼Źõ”±Ä£æé
ŗ£ŃóŹĒŅ»×ł¾Ž“óµÄ±¦²Ų£¬Čō°Ńŗ£Ė®µ»ÆŗĶ»Æ¹¤Éś²ś½įŗĻĘšĄ“£¬¼Čæɽā¾öµĖ®×ŹŌ“ȱ·¦µÄĪŹĢā£¬ÓÖæɳä·ÖĄūÓĆŗ£Ńó׏Ō“£®¶ųŅŌŗ£Ė®ĪŖÖ÷ŅŖŌĮĻµÄŗ£Ńó»Æѧ¹¤Ņµ£¬ÓÖ±»³ĘĪŖ”°Ą¶É«»Æ¹¤”±£®
£Ø1£©³£ÓƵÄŗ£Ė®µ»Æ·½·ØÓŠ
ÕōĮó
ÕōĮó
·Ø”¢
Ĥ
Ĥ
·Ø£ØµēÉųĪö”¢·“ÉųĶø£©”¢Ąä¶³·Ø”¢Ąė×Ó½»»»·ØµČ£®
£Ø2£©ČēĶ¼ŹĒµēÉųĪö·Øµ»Æŗ£Ė®µÄŌĄķĶ¼£®ĘäÖŠ£¬µē¼«A½ÓÖ±Į÷µēŌ“µÄÕż¼«£¬µē¼«B½ÓÖ±Į÷µēŌ“µÄøŗ¼«£®
¢ŁøōĤAŹĒ
ŅõĄė×Ó½»»»Ä¤
ŅõĄė×Ó½»»»Ä¤
£ØĢī£ŗŅõĄė×Ó½»»»Ä¤»ņŃōĄė×Ó½»»»Ä¤£©
¢Ś“ÓÄž²ØøŪ²É¼ÆµÄŗ£Ė®ŃłĘ·£¬¾·ÖĪöŗ¬ÓŠ“óĮæµÄNa
+ӢCl
-£¬ŅŌ¼°ÉŁĮæµÄK
+ӢSO
42-£®
ČōÓĆÉĻŹö×°ÖƶŌ²É×ŌÄž²ØøŪµÄŗ£Ė®½ųŠŠµ»Æ£¬µ±µ»Æ¹¤×÷Ķź³Éŗó£¬A”¢B”¢CČżŹŅÖŠĖłµĆČÜŅŗ£Ø»ņŅŗĢ壩µÄpH·Ö±šĪŖpH
aӢpH
bӢ
pH
c£¬ŌņĘä“óŠ”Ė³ŠņĪŖ
pHa£¼pHb£¼pHc
pHa£¼pHb£¼pHc
£®
¢ŪĒėŠ“³öÓƵēÉųĪö·Ø¶Ō²É×ŌÄž²ØøŪµÄŗ£Ė®½ųŠŠµ»Æ“¦ĄķŹ±Ėł·¢ÉśµÄ»Æѧ·“Ó¦·½³ĢŹ½
2NaCl+2H
2O
2NaOH+H
2ӟ+Cl
2ӟ
2NaCl+2H
2O
2NaOH+H
2ӟ+Cl
2ӟ
£®
£Ø3£©¾ßÓŠŌŻŹ±Ó²¶ČµÄÓ²Ė®ŌŚ³¤Ź±¼ä¼ÓČČÖó·Šŗó£¬Éś³É³ĮµķµÄÖ÷ŅŖ³É·ÖŹĒ
CaCO3”¢Mg£ØOH£©2
CaCO3”¢Mg£ØOH£©2
£®
£Ø4£©ĪŖ»ńµĆ“æ¾»Ė®£ØČ„Ąė×ÓĖ®£©£¬Ä³Ķ¬Ń§ŌŚŹµŃéŹŅ½«ŗ¬Mg
2+ӢCa
2+ӢCl
-µÄÓ²Ė®ĻČŗóĶعżŅõĄė×Ó½»»»Ź÷Ö¬[ČēRN£ØCH
3£©
3OH]ŗĶŃōĄė×Ó½»»»Ź÷Ö¬[ČēRSO
3H]£¬Š“³öCl
-ÓėÉĻŹöĄė×Ó½»»»·“Ó¦µÄ·½³ĢŹ½
RN£ØCH3£©3OH+Cl-ØTRN£ØCH3£©3Cl+OH”ą-
RN£ØCH3£©3OH+Cl-ØTRN£ØCH3£©3Cl+OH”ą-
£»½į¹ūŹµŃéĪ“»ńµĆ³É¹¦£¬ĘäæÉÄÜŌŅņŹĒ
ŅõĄė×Ó½»»»Ź÷Ö¬½»»»³öµÄOH-ŗĶMg2+”¢Ca2+µČ·“Ӧɜ³É³Įµķ¶ĀČūĮĖĄė×Ó½»»»Öł
ŅõĄė×Ó½»»»Ź÷Ö¬½»»»³öµÄOH-ŗĶMg2+”¢Ca2+µČ·“Ӧɜ³É³Įµķ¶ĀČūĮĖĄė×Ó½»»»Öł
£®









 +2NaOH
+2NaOH +NaCl+2H2O
+NaCl+2H2O +2NaOH
+2NaOH +NaCl+2H2O
+NaCl+2H2O



 £Ø2011?Äž²ØÄ£Äā£©”°»ÆѧÓė¼¼Źõ”±Ä£æé
£Ø2011?Äž²ØÄ£Äā£©”°»ÆѧÓė¼¼Źõ”±Ä£æé