
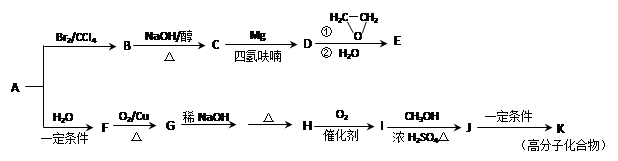
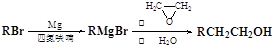
 1,2-¶žäåŅŅĶé
1,2-¶žäåŅŅĶé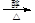 CH2="CHBr" + NaBr + H2O
CH2="CHBr" + NaBr + H2O 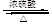
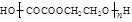 +(2n£1)H2O
+(2n£1)H2O 
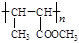
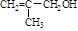
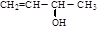
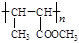 ӣ
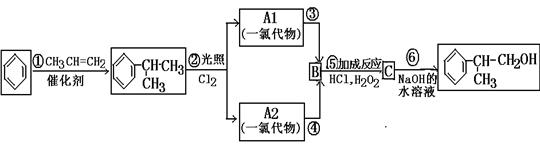
”£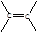 £¬BµÄĆū³ĘĪŖ1£¬2-¶žäåŅŅĶ锣
£¬BµÄĆū³ĘĪŖ1£¬2-¶žäåŅŅĶ锣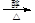 CH2="CHBr" + NaBr + H2O”£BrCH2CH2BrŗĶĒāŃõ»ÆÄĘĖ®ČÜŅŗ·¢ÉśĖ®½ā·“Ӧɜ³ÉHOCH2CH2OH£¬ĖüŗĶŅŅ¶žĖįÄÜ·¢ÉśĖõ¾Ū·“Ó¦£¬·“Ó¦·½³ĢŹ½ĪŖnHOOCCOOH+nHOCH2CH2OH
CH2="CHBr" + NaBr + H2O”£BrCH2CH2BrŗĶĒāŃõ»ÆÄĘĖ®ČÜŅŗ·¢ÉśĖ®½ā·“Ӧɜ³ÉHOCH2CH2OH£¬ĖüŗĶŅŅ¶žĖįÄÜ·¢ÉśĖõ¾Ū·“Ó¦£¬·“Ó¦·½³ĢŹ½ĪŖnHOOCCOOH+nHOCH2CH2OH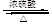
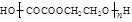 +(2n£1)H2O”£
+(2n£1)H2O”£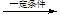
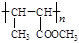 ӣ
ӣ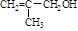 Ӣ
Ӣ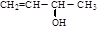 ӣ
ӣ

 »ĘøŌ¹Ś¾üæĪæĪĮ·ĻµĮŠ“š°ø
»ĘøŌ¹Ś¾üæĪæĪĮ·ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
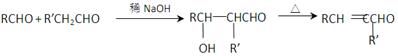
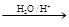 R£CH2CHO + R”äOH
R£CH2CHO + R”äOH 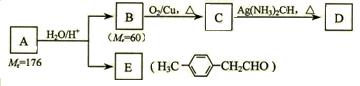
 £©µÄŅ»ĢõĀ·ĻßČēĻĀ£ŗ
£©µÄŅ»ĢõĀ·ĻßČēĻĀ£ŗ
|
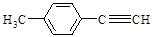
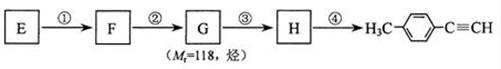 £Ø4£©Š“³öGµÄ½į¹¹¼ņŹ½£ŗ____________________________________”£
£Ø4£©Š“³öGµÄ½į¹¹¼ņŹ½£ŗ____________________________________”£| ŠņŗÅ | Ėł¼ÓŹŌ¼Į¼°·“Ó¦Ģõ¼ž | ·“Ó¦ĄąŠĶ |
| ¢Ś | ÅØ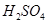 , ”÷ , ”÷ | |
| ¢Ū | Br2µÄCCl4ČÜŅŗ | |
| ¢Ü | | |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
| »ģŗĻĪļ | ³żŌÓÖŹµÄŹŌ¼Į | ³żŌÓÖŹµÄ·½·Ø |
| ±½£Ø±½·Ó£© | £Ø1£© | £Ø4£© |
| ¾Ę¾«£ØĖ®£© | £Ø2£© | £Ø5£© |
| ŅŅĖįŅŅõ„£ØŅŅĖį£© | £Ø3£© | £Ø6£© |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā

 µÄ»Æѧ·½³ĢŹ½ĪŖ
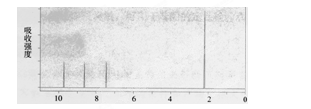
µÄ»Æѧ·½³ĢŹ½ĪŖ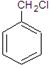 »„ĪŖĶ¬·ÖŅģ¹¹ĢåµÄ·¼Ļć×å»ÆŗĻĪļÓŠ ÖÖ£¬ĘäÖŠŅ»ÖÖĶ¬·ÖŅģ¹¹ĢåµÄŗĖ“Ź²ÕńĒāĘ×ÓŠČżÖÖĄąŠĶĒāŌ×ÓµÄĪüŹÕ·å£¬øĆĶ¬·ÖŅģ¹¹ĢåµÄ½į¹¹¼ņŹ½ĪŖ ”£
»„ĪŖĶ¬·ÖŅģ¹¹ĢåµÄ·¼Ļć×å»ÆŗĻĪļÓŠ ÖÖ£¬ĘäÖŠŅ»ÖÖĶ¬·ÖŅģ¹¹ĢåµÄŗĖ“Ź²ÕńĒāĘ×ÓŠČżÖÖĄąŠĶĒāŌ×ÓµÄĪüŹÕ·å£¬øĆĶ¬·ÖŅģ¹¹ĢåµÄ½į¹¹¼ņŹ½ĪŖ ”£²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
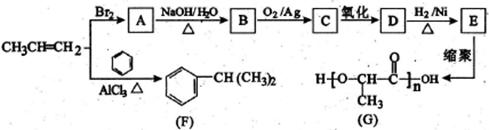
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
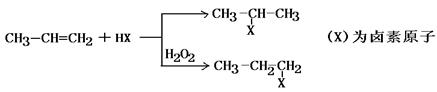

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā

 CH3COOC2H5
CH3COOC2H5²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā

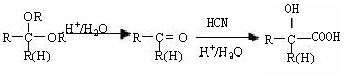
| A£®»ÆŗĻĪļII µÄ·Ö×ÓŹ½C5H9O2 |
| B£®·“Ó¦¢ÜµÄÄæµÄŹĒ·ĄÖ¹”ŖCHOŌŚµŚ¢Ż²½±»Ńõ»Æ |
| C£®·“Ó¦¢ŪŹĒĻūČ„·“Ó¦£¬·“Ó¦¢ÜŹĒõ„»Æ·“Ó¦ |
| D£®»ÆŗĻĪļD æÉŅŌ·¢ÉśĖõ¾Ū·“Ó¦µĆµ½øß·Ö×Ó»ÆŗĻĪļ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
 £¬XæÉÄÜ·¢Éś
£¬XæÉÄÜ·¢Éś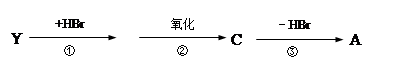
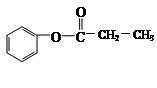 Ӣ__________________________Ӣ
”¢__________________________”¢²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com