
ijŹµŃ銔×éÉč¼ĘÓĆ50 mL 1.0 mol/LŃĪĖįøś50 mL 1.1 mol/L ĒāŃõ»ÆÄĘČÜŅŗŌŚČēĶ¼×°ÖĆÖŠ½ųŠŠÖŠŗĶ·“Ó¦”£ŌŚ“óÉÕ±µ×²æµęĖéÅŻÄĖÜĮĻ(»ņÖ½Ģõ)£¬Ź¹·ÅČėµÄŠ”ÉÕ±±æŚÓė“óÉÕ±±æŚĻąĘ½”£Č»ŗóŌŁŌŚ“󔢊”ÉÕ±Ö®¼äĢīĀśĖéÅŻÄĖÜĮĻ(»ņÖ½Ģõ)£¬“óÉÕ±ÉĻÓĆÅŻÄĖÜĮĻ°å(»ņÓ²Ö½°å)×÷øĒ°å£¬ŌŚ°åÖŠ¼äæŖĮ½øöŠ”æ×£¬ÕżŗĆŹ¹ĪĀ¶Č¼ĘŗĶ»·ŠĪ²£Į§½Į°č°ōĶعż”£Ķعż²ā¶Ø·“Ó¦¹ż³ĢÖŠĖł·Å³öµÄČČĮææɼĘĖćÖŠŗĶČČ”£ŹŌ»Ų“šĻĀĮŠĪŹĢā£ŗ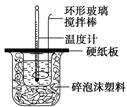
£Ø1£©±¾ŹµŃéÖŠÓĆÉŌ¹żĮæµÄNaOHµÄŌŅņ½Ģ²ÄÖŠĖµŹĒĪŖ±£Ö¤ŃĪĖįĶźČ«±»ÖŠŗĶ”£ŹŌĪŹ£ŗŃĪĖįŌŚ·“Ó¦ÖŠČōŅņĪŖÓŠ·ÅČČĻÖĻ󣬶ųŌģ³ÉÉŁĮæŃĪĖįŌŚ·“Ó¦ÖŠ»Ó·¢£¬Ōņ²āµĆµÄÖŠŗĶČČ (Ģī”°Ę«“ó”±”¢”°Ę«Š””±»ņ”°²»±ä”±)”£
£Ø2£©ŌŚÖŠŗĶČČ²ā¶ØŹµŃéÖŠ“ęŌŚÓĆĖ®Ļ“µÓĪĀ¶Č¼ĘÉĻµÄŃĪĖįµÄ²½Öč£¬ČōĪŽ“Ė²Ł×÷²½Öč£¬Ōņ²āµĆµÄÖŠŗĶČČ»į (Ģī”°Ę«“ó”±”¢”°Ę«Š””±»ņ”°²»±ä”±)”£
£Ø3£©ČōÓƵČÅØ¶ČµÄ“×ĖįÓėNaOHČÜŅŗ·“Ó¦£¬Ōņ²āµĆµÄÖŠŗĶČČ»į (Ģī”°Ę«“ó”±”¢”°Ę«Š””±»ņ”°²»±ä”±)£¬ĘäŌŅņŹĒ ”£
£Ø4£©øĆŹµŃ銔×é×öĮĖČż“ĪŹµŃ飬Ćæ“ĪČ”ČÜŅŗø÷50 mL£¬²¢¼ĒĀ¼ĻĀŌŹ¼Źż¾Ż(¼ūĻĀ±ķ)”£
| ŹµŃéŠņŗÅ | ĘšŹ¼ĪĀ¶Čt1/”ę | ÖÕÖ¹ĪĀ¶Č(t2)/”ę | ĪĀ²ī(t2£t1)/”ę | ||
| ŃĪĖį | NaOHČÜŅŗ | Ę½¾łÖµ | |||
| 1 | 25.1 | 24.9 | 25.0 | 31.6 | 6.6 |
| 2 | 25.1 | 25.1 | 25.1 | 31.8 | 6.7 |
| 3 | 25.1 | 25.1 | 25.1 | 31.9 | 6.8 |
(1)Ę«Š” (2) Ę«Š”£Ø3£©Ę«Š”£Ø4£© £56.01 kJ/mol
½āĪöŹŌĢā·ÖĪö£ŗ£Ø1£©ČōŅņĪŖÓŠ·ÅČČĻÖĻóµ¼ÖĀÉŁĮæŃĪĖįŌŚ·“Ó¦ÖŠ»Ó·¢£¬¼õÉŁĮĖHClµÄĮ棬µ¼ÖĀÉś³ÉµÄĖ®µÄĪļÖŹµÄĮæĘ«Š”£¬¹Ź²āµĆµÄÖŠŗĶČČ»įĘ«Š”£¬
£Ø2£©Ć»ÓŠÓĆĖ®Ļ“µÓĪĀ¶Č¼ĘÉĻµÄŃĪĖįČÜŅŗ£¬µ¼ÖĀŃĪĖįµÄĪļÖŹµÄĮæĘ«Š”£¬·Å³öµÄČČĮæĘ«Š”£¬²āµĆµÄÖŠŗĶČČŹżÖµĘ«Š”£¬
£Ø3£©ÓÉÓŚ“×ĖįĪŖČõĖį£¬“×ĖįµēĄėŅŖĪüŹÕÄÜĮ棬Ōģ³É²āµĆµÄÖŠŗĶČČĘ«Š”£¬
¹Ź“š°øĪŖ£ŗĘ«Š”£»ÓĆ“×Ėį“śĢęŃĪĖį£¬“×ĖįµēĄėŅŖĪüŹÕÄÜĮ棬Ōģ³É²āµĆµÄÖŠŗĶČČĘ«Š”£»
£Ø4£©±ķÖŠČż“Ī²āĮæŹż¾Ż¶¼ŹĒÓŠŠ§µÄ£¬Čż“ĪĪĀ²īµÄĘ½¾łÖµĪŖ£ŗ
¦¤H£½£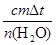 £½£
£½£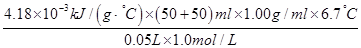 £½£56.01 kJ/mol”£
£½£56.01 kJ/mol”£
øł¾ŻÖŠŗĶČȵÄøÅÄīæÉÖŖ£¬ČČ»Æѧ·½³ĢŹ½ĪŖ£ŗHCl£Øaq£©+NaOH£Øaq£©=NaCl£Øaq£©+H2O£Øl£©”÷H=-57.3KJ/mol£»
¹Ź“š°øĪŖ£ŗ-56.01 kJ/mol£»HCl£Øaq£©+NaOH£Øaq£©=NaCl£Øaq£©+H2O£Øl£©”÷H=-57.3KJ/mol£®
æ¼µć£ŗ±¾Ģāæ¼²éĮĖÖŠŗĶČČµÄ³Įµķ


 ŌĶĮæģ³µĻµĮŠ“š°ø
ŌĶĮæģ³µĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
¼×“¼ŹĒŅ»ÖÖ³£ÓƵÄČ¼ĮĻ£¬¹¤ŅµÉĻæÉŅŌÓĆCOŗĶH2ŌŚŅ»¶ØĢõ¼žĻĀŗĻ³É¼×“¼”£
£Ø1£©ŅŃÖŖCO£Øg£©”¢H2£Øg£©”¢CH3OH£Ø1£©µÄČ¼ÉÕČČ”÷H·Ö±šĪŖ£ŗ-283.0kJ£Æmol”¢-285.8 kJ/mol”¢-726.5kJ/mol£¬ŌņCOŗĻ³É¼×“¼µÄČČ»Æѧ·½³ĢŹ½ĪŖ£ŗ ”£
£Ø2£©ŌŚŗćČŻĆܱÕČŻĘ÷ÖŠCOÓėH2·¢Éś·“Ӧɜ³É¼×“¼£¬ø÷ĪļÖŹÅضČŌŚ²»Ķ¬Ģõ¼žĻĀµÄ±ä»ÆדæöČēĶ¼ĖłŹ¾£ØæŖŹ¼Ź±ĒāĘųµÄÅضČĒśĻßŗĶ8·ÖÖÓŗó¼×“¼µÄÅضČĒśĻßĪ“»³ö”£4·ÖÖÓŗĶ8·ÖÖÓøıäµÄĢõ¼ž²»Ķ¬£©£ŗ 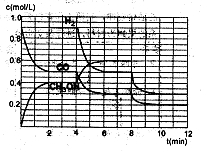
¢ŁĻĀĮŠĖµ·ØÕżČ·µÄŹĒ
| A£®ĘšŹ¼Ź±n£ØH2£©ĪŖ1£®7mol |
| B£®µ±ČŻĘ÷ÄŚŃ¹Ēæŗć¶ØŹ±£¬ĖµĆ÷·“Ó¦“ļµ½Ę½ŗāדĢ¬ |
| C£®4·ÖÖÓŹ±£¬øıäµÄĢõ¼žŹĒÉżøßĪĀ¶Č |
| D£®7·ÖÖÓŹ±£¬v£ØCO£©=v£ØCH3OH£© |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¼ĘĖćĢā
£Ø1£©H2SµÄČ¼ÉÕČȦ¤H£½ £a kJ”¤mol£1£¬ŌņH2SČ¼ÉÕ·“Ó¦µÄČČ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø2£©ŅŃÖŖ£ŗøßĪĀĻĀ£¬ŌŚĆܱÕČŻĘ÷ÖŠÓĆH2»¹ŌWO2æɵƵ½½šŹōĪŁ”£µ±ĪĀ¶Č¹żøߏ±£¬WO2(s)»į×Ŗ±äĪŖWO2 (g)”£Ēėøł¾ŻŅŌĻĀ·“Ó¦£ŗ
WO2 (s) + 2H2 (g)  W (s) + 2H2O (g)£»¦¤H£½ +66.0 kJ”¤ mol£1
W (s) + 2H2O (g)£»¦¤H£½ +66.0 kJ”¤ mol£1
WO2 (g) + 2H2 W (s) + 2H2O (g)£»¦¤H £½ £137.9 kJ”¤ mol£1
W (s) + 2H2O (g)£»¦¤H £½ £137.9 kJ”¤ mol£1
¼ĘĖć³öWO2 (s)  WO2 (g) µÄ¦¤H £½ ______________________”£
WO2 (g) µÄ¦¤H £½ ______________________”£
£Ø3£©¹¤ŅµÉĻ³£ĄūÓĆĢģČ»Ęų(Ö÷ŅŖ³É·ÖĪŖCH4)ÓėCO2½ųŠŠøßĪĀÖŲÕūÖʱøCO£¬·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ£ŗ
CH4 + CO2 =" 2CO" + 2H2
ŅŃÖŖCH4”¢H2ŗĶCOµÄČ¼ÉÕČČ·Ö±šĪŖ890.3 kJ”¤mol-1”¢285.8 kJ”¤mol-1”¢283.0 kJ”¤ mol-1£¬ŌņÉś³É1 m3(±ź×¼×“æö)COĖłŠčČČĮæĪŖ £»
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¼ĘĖćĢā
¹¤ŅµŗĻ³É°±µÄ·“Ó¦ĪŖ£ŗN2(g)+3H2(g)  2NH3(g) ”÷H<0”£Ä³ŹµŃ齫3.0 mol N2(g)ŗĶ4. 0 mol H2(g)³äČėČŻ»żĪŖ10LµÄĆܱÕČŻĘ÷ÖŠ£¬ŌŚĪĀ¶ČT1ĻĀ·“Ó¦”£²āµĆH2µÄĪļÖŹµÄĮæĖę·“Ó¦Ź±¼äµÄ±ä»ÆČēĻĀĶ¼ĖłŹ¾”£
2NH3(g) ”÷H<0”£Ä³ŹµŃ齫3.0 mol N2(g)ŗĶ4. 0 mol H2(g)³äČėČŻ»żĪŖ10LµÄĆܱÕČŻĘ÷ÖŠ£¬ŌŚĪĀ¶ČT1ĻĀ·“Ó¦”£²āµĆH2µÄĪļÖŹµÄĮæĖę·“Ó¦Ź±¼äµÄ±ä»ÆČēĻĀĶ¼ĖłŹ¾”£
£Ø1£©·“Ó¦æŖŹ¼3minÄŚ£¬H2µÄĘ½¾ł·“Ó¦ĖŁĀŹĪŖ ”£
£Ø2£©¼ĘĖćøĆĢõ¼žĻĀŗĻ³É°±·“Ó¦µÄ»ÆŃ§Ę½ŗā³£Źż£ØŠ“³ö¼ĘĖć¹ż³Ģ£¬½į¹ū±£Įō2Ī»ÓŠŠ§Źż×Ö£©”£
£Ø3£©½öøıäĪĀ¶ČĪŖT2 ( T2Š”ÓŚTI£©ŌŁ½ųŠŠŹµŃ飬ĒėŌŚ“šĢāæØæņĶ¼ÖŠ»³öH2µÄĪļÖŹµÄĮæĖę
·“Ó¦Ź±¼ä±ä»ÆµÄŌ¤ĘŚ½į¹ūŹ¾ŅāĶ¼”£
£Ø4£©ŌŚŅŌĆŗĪŖÖ÷ŅŖŌĮĻµÄŗĻ³É°±¹¤ŅµÖŠ£¬ŌĮĻĘųĒāĘų³£ÓĆĻĀŹö·½·Ø»ńµĆ£ŗ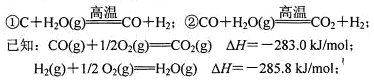
Š“³öÉĻŹöCOÓėH2O(g)·“Ó¦µÄČČ»Æѧ·½³ĢŹ½£ŗ ”£
£Ø5£©ŗĻ³É°±¹¤ŅµÖŠ£¬ŌĮĻĘų(N2”¢H2»ģÓŠÉŁĮæCO”¢NH3£©ŌŚ½ųČėŗĻ³ÉĖžÖ®Ē°£¬ÓĆ“×Ėį¶ž°±ŗĻĶ£ØI£©ČÜŅŗĄ“ĪüŹÕCOĘä·“Ó¦ĪŖ£ŗCH3COO[Cu(NH3)2]+CO+NH3 CH3COO[Cu(NH3)3]?CO ”÷H<0”£Š“³öĢįøßCOĪüŹÕĀŹµÄĘäÖŠŅ»Ļī“ėŹ©£ŗ ”£
CH3COO[Cu(NH3)3]?CO ”÷H<0”£Š“³öĢįøßCOĪüŹÕĀŹµÄĘäÖŠŅ»Ļī“ėŹ©£ŗ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¼ĘĖćĢā
ŅŅ“¼ĘūÓĶŹĒ±»¹ć·ŗŹ¹ÓƵĊĀŠĶĒå½ąČ¼ĮĻ£¬¹¤ŅµÉś²śŅŅ“¼µÄŅ»ÖÖ·“Ó¦ŌĄķĪŖ£ŗ
2CO(g) + 4H2(g) CH3CH2OH(g) + H2O(g) ”÷H =" ”Ŗ256.1" kJ”¤mol£1
CH3CH2OH(g) + H2O(g) ”÷H =" ”Ŗ256.1" kJ”¤mol£1
ŅŃÖŖ£ŗCO(g) + H2O(g) CO2(g)+H2(g) ”÷H=" ”Ŗ41.2" kJ”¤mol£1
CO2(g)+H2(g) ”÷H=" ”Ŗ41.2" kJ”¤mol£1
£Ø1£©ŅŌCO2(g)ÓėH2(g)ĪŖŌĮĻŅ²æÉŗĻ³ÉŅŅ“¼£¬ĘäČČ»Æѧ·½³ĢŹ½ČēĻĀ£ŗ
2CO2(g) +6H2(g) CH3CH2OH(g) +3H2O(g) ”÷H = ”£
CH3CH2OH(g) +3H2O(g) ”÷H = ”£
£Ø2£©Ęū³µŹ¹ÓĆŅŅ“¼ĘūÓĶ²¢²»ÄܼõÉŁNOxµÄÅÅ·Å£¬ÕāŹ¹NOxµÄÓŠŠ§Ļū³ż³ÉĪŖ»·±£ĮģÓņµÄÖŲŅŖæĪĢā”£
¢ŁÄ³ŃŠ¾æŠ”×éŌŚŹµŃéŹŅŅŌAgØC ZSMØC 5ĪŖ“߻ƼĮ£¬²āµĆNO×Ŗ»ÆĪŖN2µÄ×Ŗ»ÆĀŹĖęĪĀ¶Č±ä»ÆĒéæöČēĻĀĶ¼”£Čō²»Ź¹ÓĆCO£¬ĪĀ¶Č³¬¹ż800”ę£¬·¢ĻÖNOµÄ×Ŗ»ÆĀŹ½µµĶ£¬ĘäæÉÄܵÄŌŅņĪŖ £»ŌŚn(NO)/n(C O)=1µÄĢõ¼žĻĀ£¬Ó¦æŲÖʵÄ×ī¼ŃĪĀ¶ČŌŚ ×óÓŅ”£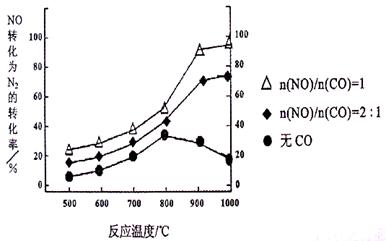
¢ŚÓĆ»īŠŌĢ滹Ō·Ø“¦ĄķµŖŃõ»ÆĪļ”£ÓŠ¹Ų·“Ó¦ĪŖ£ŗC (s) +2NO2(g) N2 (g) + CO2 (g)”£Ä³ŃŠ¾æŠ”×éĻņijĆܱÕČŻĘ÷ÖŠ¼ÓČĖ×ćĮæµÄ»īŠŌĢæŗĶNO£¬ŗćĪĀ( T1”ę)Ģõ¼žĻĀ·“Ó¦£¬·“Ó¦½ųŠŠµ½²»Ķ¬Ź±¼ä²āµĆø÷ĪļÖŹµÄÅضČČēĻĀ£ŗ
N2 (g) + CO2 (g)”£Ä³ŃŠ¾æŠ”×éĻņijĆܱÕČŻĘ÷ÖŠ¼ÓČĖ×ćĮæµÄ»īŠŌĢæŗĶNO£¬ŗćĪĀ( T1”ę)Ģõ¼žĻĀ·“Ó¦£¬·“Ó¦½ųŠŠµ½²»Ķ¬Ź±¼ä²āµĆø÷ĪļÖŹµÄÅضČČēĻĀ£ŗ
| ”” ÅضČ/mol?L£1 Ź±¼ä/min | NO | N2 | CO2 |
| 0 | 1.00 | 0 | 0 |
| 20 | 0.40 | 0.30 | 0.30 |
| 30 | 0.40 | 0.30 | 0.30 |
| 40 | 0.32 | 0.34 | 0.17 |
| 50 | 0.32 | 0.34 | 0.17 |

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
(14 ·Ö) Ņ»Ńõ»ÆĢ¼±»¹ć·ŗÓ¦ÓĆÓŚŅ±½š¹¤ŅµŗĶµē×Ó¹¤Ņµ”£
¢ÅøßĀÆĮ¶ĢśŹĒ×īĪŖĘÕ±éµÄĮ¶Ģś·½·Ø£¬Ļą¹Ų·“Ó¦µÄČČ»Æѧ·½³ĢŹ½ČēĻĀ£ŗ
4CO(g)£«Fe3O4(s)£½4CO2(g)£«3Fe(s) ”÷H="a" kJ”¤mol£1
CO(g)£«3Fe2O3(s)£½CO2(g)£«2Fe3O4(s) ”÷H="b" kJ”¤mol£1
·“Ó¦3CO(g)£«Fe2O3(s)£½3CO2(g)£«2Fe(s)µÄ”÷H= kJ”¤mol£1(ÓĆŗ¬a”¢b µÄ“śŹżŹ½±ķŹ¾)”£
¢Ęµē×Ó¹¤ŅµÖŠŹ¹ÓƵÄŅ»Ńõ»ÆĢ¼³£ŅŌ¼×“¼ĪŖŌĮĻĶعżĶŃĒā”¢·Ö½āĮ½²½·“Ó¦µĆµ½”£
µŚŅ»²½£ŗ2CH3OH(g) HCOOCH3(g)+2H2(g) ”÷H>0
HCOOCH3(g)+2H2(g) ”÷H>0
µŚ¶ž²½£ŗHCOOCH3(g) CH3OH(g) +CO(g) ”÷H>0
CH3OH(g) +CO(g) ”÷H>0
¢ŁµŚŅ»²½·“Ó¦µÄ»śĄķæÉŅŌÓĆĻĀĶ¼±ķŹ¾£ŗ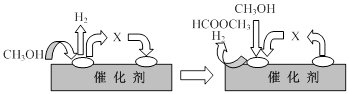
Ķ¼ÖŠÖŠ¼ä²śĪļXµÄ½į¹¹¼ņŹ½ĪŖ ”£
¢ŚŌŚ¹¤ŅµÉś²śÖŠ£¬ĪŖĢįøßCOµÄ²śĀŹ£¬æɲÉČ”µÄŗĻĄķ“ėŹ©ÓŠ ”£
¢ĒĪŖ½ųŠŠĻą¹ŲŃŠ¾æ£¬ÓĆCO»¹ŌøßĀĮĢśæóŹÆ£¬·“Ó¦ŗó¹ĢĢåĪļÖŹµÄX”ŖÉäĻßŃÜÉäĘ×Ķ¼ČēĶ¼ĖłŹ¾£ØX”ŖÉäĻßŃÜÉäæÉÓĆÓŚÅŠ¶Ļij¾§Ģ¬ĪļÖŹŹĒ·ń“ęŌŚ£¬²»Ķ¬¾§Ģ¬ĪļÖŹ³öĻÖŃÜÉä·åµÄŃÜÉä½Ē²»Ķ¬£©”£·“Ó¦ŗó»ģŗĻĪļÖŠµÄŅ»ÖÖ²śĪļÄÜÓėŃĪĖį·“Ӧɜ²śĮ½ÖÖŃĪ£¬øĆ·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ ”£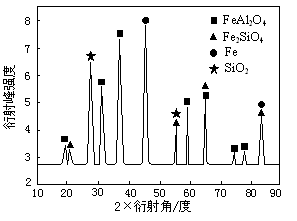
¢Čij“߻ƼĮѳʷ£Øŗ¬Ni2O340%£¬ĘäÓąĪŖSiO2£©Ķعż»¹Ō”¢Ģį“æĮ½²½»ńµĆÄųµ„ÖŹ£ŗŹ×ĻČÓĆCO½«33.2 gѳʷŌŚ¼ÓČČĢõ¼žĻĀ»¹ŌĪŖ“ÖÄų£»Č»ŗóŌŚ³£ĪĀĻĀŹ¹“ÖÄųÖŠµÄNiÓėCO½įŗĻ³ÉNi(CO)4£Ø·Šµć43 ”ę£©£¬²¢ŌŚ180 ”ꏱŹ¹Ni(CO)4ÖŲŠĀ·Ö½ā²śÉśÄųµ„ÖŹ”£
ÉĻŹöĮ½²½ÖŠĻūŗÄCOµÄĪļÖŹµÄĮæÖ®±ČĪŖ ”£
¢ÉĪŖ°²Č«Ęš¼ū£¬¹¤ŅµÉś²śÖŠŠč¶ŌæÕĘųÖŠµÄCO½ųŠŠ¼ą²ā”£
¢Ł·ŪŗģÉ«µÄPdCl2ČÜŅŗæÉŅŌ¼ģŃéæÕĘųÖŠÉŁĮæµÄCO”£ČōæÕĘųÖŠŗ¬CO£¬ŌņČÜŅŗÖŠ»į²śÉśŗŚÉ«µÄPd³Įµķ”£ĆæÉś³É5.3gPd³Įµķ£¬·“Ó¦×ŖŅʵē×ÓŹżĪŖ ”£
¢ŚŹ¹ÓƵē»ÆѧŅ»Ńõ»ÆĢ¼ĘųĢå“«øŠĘ÷¶ØĮæ¼ģ²āæÕĘųÖŠCOŗ¬Į棬Ęä½į¹¹ČēĶ¼ĖłŹ¾”£ÕāÖÖ“«øŠĘ÷ĄūÓĆŌµē³ŲŌĄķ£¬ŌņøƵē³ŲµÄøŗ¼«·“Ó¦Ź½ĪŖ ”£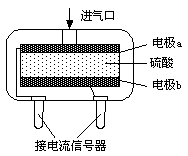
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
50mL0.50mol/LŃĪĖįÓė50mL0.55mol/LNaOHČÜŅŗŌŚČēĶ¼ĖłŹ¾µÄ×°ÖĆÖŠ½ųŠŠÖŠŗĶ·“Ó¦”£Ķعż²ā¶Ø·“Ó¦¹ż³ĢÖŠĖł·Å³öµÄČČĮææɼĘĖćÖŠŗĶČČ”£»Ų“šĻĀĮŠĪŹĢā£ŗ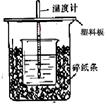
£Ø1£©“ÓŹµŃé×°ÖĆÉĻæ“£¬Ķ¼ÖŠÉŠČ±ÉŁµÄŅ»ÖÖ²£Į§ÓĆĘ·_____ ___”£
£Ø2£©ÉÕ±¼äĢīĀśĖéÖ½ĢõµÄ×÷ÓĆŹĒ___________________________”£
£Ø3£©“óÉÕ±ÉĻČē²»øĒÓ²Ö½°å£¬ĒóµĆµÄÖŠŗĶČČŹżÖµ £ØĢī”°Ę«“ó”±”¢”°Ę«Š””±”¢”°ĪŽ”°Ó°Ļģ”±£©”£
£Ø4£©ŹµŃéÖŠ60mL0.50mol/LŃĪĖįÓė50mL0.55mol/LNaOHČÜŅŗ½ųŠŠ·“Ó¦£¬ÓėÉĻŹöŹµŃéĻą±Č£¬Ėł·Å³öµÄČČĮæ £ØĢī”°ĻąµČ”±”¢”°²»ĻąµČ”±£©£¬ĖłĒóÖŠŗĶČČ £ØĢī”°ĻąµČ”±”¢”°²»ĻąµČ”±£©£¬¼ņŹöĄķÓÉ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗµ„Ń”Ģā
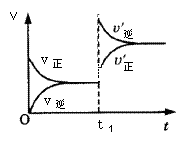
£ķ£Į£Ø£ó£©£«£ī£Ā£Ø£ē£©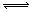 £ń£Ć£Ø£ē£©µÄæÉÄę·“Ó¦ÖŠ£¬Ķ¼¼×ŌŚŗćĪĀĢõ¼ž“ļĘ½ŗāŹ±£¬BµÄĢå»ż·ÖŹż£Ø£Ā£„£©ÓėŃ¹Ēæ£Øp£©µÄ¹ŲĻµ£¬Ķ¼ŅŅ±ķŹ¾ŌŚŅ»¶ØĢõ¼žĻĀ“ļµ½Ę½ŗā£Ø£öÕż£½£öÄę£©ŗó£ō1Ź±æĢøıäÓ°ĻģĘ½ŗāµÄĮķŅ»øöĢõ¼žÖŲŠĀ½ØĮ¢ŠĀĘ½ŗāµÄ·“Ó¦¹ż³Ģ£¬ÓŠ¹ŲŠšŹöÕżČ·µÄŹĒ
£ń£Ć£Ø£ē£©µÄæÉÄę·“Ó¦ÖŠ£¬Ķ¼¼×ŌŚŗćĪĀĢõ¼ž“ļĘ½ŗāŹ±£¬BµÄĢå»ż·ÖŹż£Ø£Ā£„£©ÓėŃ¹Ēæ£Øp£©µÄ¹ŲĻµ£¬Ķ¼ŅŅ±ķŹ¾ŌŚŅ»¶ØĢõ¼žĻĀ“ļµ½Ę½ŗā£Ø£öÕż£½£öÄę£©ŗó£ō1Ź±æĢøıäÓ°ĻģĘ½ŗāµÄĮķŅ»øöĢõ¼žÖŲŠĀ½ØĮ¢ŠĀĘ½ŗāµÄ·“Ó¦¹ż³Ģ£¬ÓŠ¹ŲŠšŹöÕżČ·µÄŹĒ
¼× ŅŅ
| A£®n<q | B£®n>q |
| C£®Õż·“Ó¦ĪŖ·ÅČČ·“Ó¦ | D£®Xµć±ČYµć·“Ó¦ĖŁĀŹæģ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗµ„Ń”Ģā
Ņ»¶ØĢõ¼žĻĀ£¬ŌŚĢå»żĪŖ10LµÄĆܱÕČŻĘ÷ÖŠ£¬1 molXŗĶ1 molY·¢Éś·“Ó¦£ŗ2x£Øg£©+Y£Øg£© Z£Øg£©£¬¾60s“ļµ½Ę½ŗā£¬Éś³É0£®3mol Z”£ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ
Z£Øg£©£¬¾60s“ļµ½Ę½ŗā£¬Éś³É0£®3mol Z”£ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ
| A£®·“Ó¦½ųŠŠ30 sŹ±£¬Õż·“Ó¦ĖŁĀŹµČÓŚÄę·“Ó¦ĖŁĀŹ |
| B£®·“Ó¦½ųŠŠ80 sŹ±£¬Äę·“Ó¦ĖŁĀŹ“óÓŚÕż·“Ó¦ĖŁĀŹ |
| C£®·“Ó¦½ųŠŠ60 sŹ±£¬XµÄĪļÖŹµÄĮæÅضČĪŖ0£®04 mol/L |
| D£®·“Ó¦½ųŠŠ60 sŹ±£¬YµÄ×Ŗ»ÆĀŹĪŖ70£„ |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com