
 ʵ����������0.1mol/L NaOH��Һ�������й�����ʵ�飬��ݴ˻ش���������
ʵ����������0.1mol/L NaOH��Һ�������й�����ʵ�飬��ݴ˻ش���������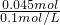 =0.45L=450mL��
=0.45L=450mL�� ����������������ҺŨ�ȵ�Ӱ�죻
����������������ҺŨ�ȵ�Ӱ�죻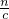 ��������������Һ�������
��������������Һ������� ������Һ����������������5������������ˮ����Ӧʵ��������ˮ��Ӧ���������������������������������������Ʒ�Ӧ�������ǻ��������ƣ�
������Һ����������������5������������ˮ����Ӧʵ��������ˮ��Ӧ���������������������������������������Ʒ�Ӧ�������ǻ��������ƣ� 


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ʵ����������0.1mol/L NaOH��Һ�������й�����ʵ�飬��ݴ˻ش���������
ʵ����������0.1mol/L NaOH��Һ�������й�����ʵ�飬��ݴ˻ش����������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʵ����������0.1mol/L NaOH��Һ�������й�����ʵ�飬��ݴ˻ش���������
��1��ʵ��ʱ��������������Һ500mL��Ӧ��������ƽ��ȡ���������Ϊ g��
��2��������NaOH��Һʱ���õIJ���������Ҫ����Ͳ���ձ����������� �� ��
��3����ʵ��������������������������������Һ��Ũ��ƫ�͵���_______��������ĸ��
a.��ˮʱԽ���̶���
b.δϴ���ձ��Ͳ�����
c.����ƿ�ڱڸ���ˮ���δ���ﴦ��
d.����ʱ���ӿ̶���
��4����VmL����Ũ�ȵ�NaOH��Һ�У�ͨ��һ������CO2��Ȼ�������Һ����μ���1mol![]() L-1�����ᣬ������������Һ����������CO2�������ϵ����ͼ��ʾ��
L-1�����ᣬ������������Һ����������CO2�������ϵ����ͼ��ʾ��

�ٵ�����35mL������Һʱ������������̼�����ʵ���Ϊ__________mol��
��д������Ũ�ȵ�NaOH��Һͨ��CO2�μ�����֮ǰ��������Һ������Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��ɽ��ʡ�������ϣ����л�ѧ�Ծ��������棩 ���ͣ������

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com