
��13�֣�ijѧϰС��������кͷ�Ӧԭ����DISϵͳ�������ֻ���Ϣϵͳ���ɴ����������ݲɼ����ͼ������ɣ��ⶨij��ˮ�����ʵ���Ũ�ȣ��Բ�����Һ�����������жϵζ��յ㡣��ش�
��1��ʵ��ʱ��ȡ10.00mL��ˮ��Ʒ�����Ƴ�100mL���ⰱˮ����ò���������Ҫ��������_________________�����������ƣ���
��2����ȡ20.00mL������Һ����ྻ������ƿ�У����Ӻ�DISϵͳ�������ƿ�к�����������ˮ���Ƿ��Ӱ��������_______����ǡ���������ȷ��������
��3���ٵζ���ʢ������ǰ��Ҫ��__________________������������ˮϴ�Ӻ���_________________��Ȼ������������Һ������Һ�����͵㴦�ڵζ��ܵ�_________________��
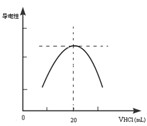
������ƿ�еμ�0.10mol��L��1�����ᣬ�������Ļ����ʾ����Һ����������������������ϵ������ͼ��

д����ˮ�����ᷴӦ�����ӷ���ʽ___________________________���ð�ˮ��Ʒ�����ʵ���Ũ��Ϊ_____________��
��4����һѧϰС������Ϊ������������Դ���һ������Ϊ���ɵ�NH4Cl ��ǿ�������Σ��ᷢ��ˮ���ʹNH4+ Ũ���½���������ǡ����ȫ��ӦʱNH4+ Ũ�Ȳ������ֵ����Һ�����ԾͲ������ֵ������Ϊ��ѧϰС��Ľ����Ƿ���ȷ��________��������___________________��
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ijѧϰС��������кͷ�Ӧԭ����DISϵͳ�������ֻ���Ϣϵͳ���ɴ����������ݲɼ����ͼ������ɣ��ⶨij��ˮ�����ʵ���Ũ�ȣ��Բ�����Һ�����������жϵζ��յ㣮��ش�
ijѧϰС��������кͷ�Ӧԭ����DISϵͳ�������ֻ���Ϣϵͳ���ɴ����������ݲɼ����ͼ������ɣ��ⶨij��ˮ�����ʵ���Ũ�ȣ��Բ�����Һ�����������жϵζ��յ㣮��ش��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��13�֣�ijѧϰС��������кͷ�Ӧԭ����DISϵͳ�������ֻ���Ϣϵͳ���ɴ����������ݲɼ����ͼ������ɣ��ⶨij��ˮ�����ʵ���Ũ�ȣ��Բ�����Һ�����������жϵζ��յ㡣��ش�
��1��ʵ��ʱ��ȡ10.00mL��ˮ��Ʒ�����Ƴ�100mL���ⰱˮ����ò���������Ҫ��������_________________�����������ƣ���
��2����ȡ20.00mL������Һ����ྻ������ƿ�У����Ӻ�DISϵͳ�������ƿ�к�����������ˮ���Ƿ��Ӱ��������_______����ǡ���������ȷ��������
��3���ٵζ���ʢ������ǰ��Ҫ��__________________������������ˮϴ�Ӻ���_________________��Ȼ������������Һ������Һ�����͵㴦�ڵζ��ܵ�_________________��
������ƿ�еμ�0.10mol��L��1�����ᣬ�������Ļ����ʾ����Һ����������������������ϵ������ͼ��
д����ˮ�����ᷴӦ�����ӷ���ʽ___________________________���ð�ˮ��Ʒ�����ʵ���Ũ��Ϊ_____________��
��4����һѧϰС������Ϊ������������Դ���һ������Ϊ���ɵ�NH4Cl ��ǿ�������Σ��ᷢ��ˮ���ʹNH4+ Ũ���½���������ǡ����ȫ��ӦʱNH4+ Ũ�Ȳ������ֵ����Һ�����ԾͲ������ֵ������Ϊ��ѧϰС��Ľ����Ƿ���ȷ��________��������___________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ����ױ�������һ�и߶���ѧ���������ƿ��Ի�ѧ�Ծ����������� ���ͣ�ʵ����
(��14��)ijѧϰС��������кͷ�Ӧԭ����DISϵͳ�������ֻ���Ϣϵͳ���ɴ����������ݲɼ����ͼ������ɣ��ⶨij��ˮ�����ʵ���Ũ�ȣ��Բ�����Һ�����������жϵζ��յ㡣��ش�
��1��ʵ��ʱ��ȡ10.00mL��ˮ��Ʒ�����Ƴ�100mL���ⰱˮ��
��2����ȡ20.00mL������Һ����ྻ������ƿ�У����Ӻ�DISϵͳ�������ƿ�к�����������ˮ���Ƿ��Ӱ��������_____________________����ǡ���������ȷ��������
��3���ٵζ���ʢ������ǰ��Ҫ��__________________________________________������������ˮϴ�Ӻ���__________________________________________________��Ȼ������������Һ������Һ�����͵㴦�ڵζ��ܵ�_____________________________________��
������ƿ�еμ�0.10mol��L��1�����ᣬ�������Ļ����ʾ����Һ����������������������ϵ������ͼ��
д����ˮ�����ᷴӦ�����ӷ���ʽ___________________________________________���ð�ˮ��Ʒ�����ʵ���Ũ��Ϊ__________________________�����ڵζ������в�����������Һ������ƿ�⣬���ʹ�ⶨ��� ������ƫ�ߡ�ƫ�͡���Ӱ�죩
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014����ױ�������һ�и߶���ѧ���������ƿ��Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
(��14��)ijѧϰС��������кͷ�Ӧԭ����DISϵͳ�������ֻ���Ϣϵͳ���ɴ����������ݲɼ����ͼ������ɣ��ⶨij��ˮ�����ʵ���Ũ�ȣ��Բ�����Һ�����������жϵζ��յ㡣��ش�
��1��ʵ��ʱ��ȡ10.00mL��ˮ��Ʒ�����Ƴ�100mL���ⰱˮ��
��2����ȡ20.00mL������Һ����ྻ������ƿ�У����Ӻ�DISϵͳ�������ƿ�к�����������ˮ���Ƿ��Ӱ��������_____________________����ǡ���������ȷ��������
��3���ٵζ���ʢ������ǰ��Ҫ��__________________________________________������������ˮϴ�Ӻ���__________________________________________________��Ȼ������������Һ������Һ�����͵㴦�ڵζ��ܵ�_____________________________________��
������ƿ�еμ�0.10mol��L��1�����ᣬ�������Ļ����ʾ����Һ����������������������ϵ������ͼ��
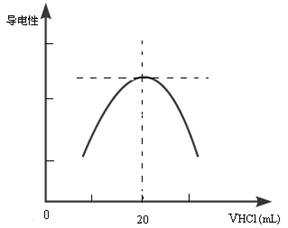
д����ˮ�����ᷴӦ�����ӷ���ʽ___________________________________________���ð�ˮ��Ʒ�����ʵ���Ũ��Ϊ__________________________�����ڵζ������в�����������Һ������ƿ�⣬���ʹ�ⶨ��� ������ƫ�ߡ�ƫ�͡���Ӱ�죩
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com