
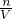 ����������������ҺŨ�ȵ�Ӱ�죮
����������������ҺŨ�ȵ�Ӱ�죮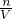 ������Һ����������Ӧ��ע�⣨1�����Ȼ��Ƶ���������ƽ�ľ���Ϊ0.1��
������Һ����������Ӧ��ע�⣨1�����Ȼ��Ƶ���������ƽ�ľ���Ϊ0.1�� 

 ʱ�����������ҵԭ���ܳ�����ϵ�д�
ʱ�����������ҵԭ���ܳ�����ϵ�д� ����νӽ̲���ĩ���Ԥϰ�人������ϵ�д�
����νӽ̲���ĩ���Ԥϰ�人������ϵ�д� ������ҵ��ٳɳ����½������������ϵ�д�
������ҵ��ٳɳ����½������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014��㶫ʡ��һ��ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
��13�֣���֪ij��ҵ��ˮ�к��д���FeSO4���϶��Cu2+��������Na+ �Լ��������࣬ͨ���������̿ɴӸ÷�ˮ�л���FeSO4��7H2O���弰����Cu��
��1������1����Ҫ������ �����õ��IJ��������� , _______�� ��
��2������2�з�����Ӧ�����ӷ���ʽΪ
��3������3�м����Y��Һ�� ���ѧʽ��
��4������4���漰�IJ����ǣ�����Ũ���� �����ˣ�ϴ�ӣ���ɡ�
��5��ʵ����Ҫ����100mL1.0mol��L��1 FeSO4��Һ�������㣬Ӧ����������ƽ��ȡFeSO4��7H2O���� g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com