
·ÖĪö Ņ»·Ż¼ÓČė×ćĮæNaOHČÜŅŗ£¬Éś³ÉĄ¶É«³Įµķ£¬½«“ĖŠü×ĒŅŗ¼ÓČČ£¬ŅŻ³öµÄĘųĢåÄÜŹ¹ŹŖČóµÄŗģÉ«ŹÆČļŹŌÖ½±äĄ¶£¬ĖµĆ÷Éś³É°±Ęų£¬ŃōĄė×Óŗ¬ÓŠNH4+£¬ĒŅn£ØNH4+£©=n£ØNH3£©=n£ØHCl£©=0.05L”Į1mol/L=0.05mol£¬Ą¶É«³ĮµķĪŖĒāŃõ»ÆĶ£¬ĖµĆ÷ŗ¬ÓŠCu2+£¬ĮķŅ»·Ż¼ÓČė×ćĮæµÄBaCl2ČÜŅŗ£¬Éś³É²»ČÜÓŚĻ”ĻõĖį°×É«³Įµķ£¬¾¹żĀĖ”¢Ļ“µÓ”¢øÉŌļ³ĘĘäÖŹĮæĪŖ11.65g£¬Ó¦ĪŖBaSO4£¬æÉÖŖn£ØSO42-£©=n£ØBaSO4£©=$\frac{11.65g}{233g/mol}$=0.05mol£¬ŅŌ“ĖæÉČ·¶Øn£Ø£ØNH4£©2SO4£©£ŗ£ØCuSO4£©£¬½įŗĻÖŹĮæČ·¶ØĖ®µÄĮ棬æÉČ·¶Ø»ÆѧŹ½£®
½ā“š ½ā£ŗ£Ø1£©Ņ»·Ż¼ÓČė×ćĮæNaOHČÜŅŗ£¬Éś³ÉĄ¶É«³Įµķ£¬½«“ĖŠü×ĒŅŗ¼ÓČČ£¬ŅŻ³öµÄĘųĢåÄÜŹ¹ŹŖČóµÄŗģÉ«ŹÆČļŹŌÖ½±äĄ¶£¬ĖµĆ÷Éś³É°±Ęų£¬ŃōĄė×Óŗ¬ÓŠNH4+£¬Ą¶É«³ĮµķĪŖĒāŃõ»ÆĶ£¬ĖµĆ÷ŗ¬ÓŠCu2+£¬ĮķŅ»·Ż¼ÓČė×ćĮæµÄBaCl2ČÜŅŗ£¬Éś³É²»ČÜÓŚĻ”ĻõĖį°×É«³Įµķ£¬Ó¦ĪŖBaSO4£¬ĖµĆ÷ŗ¬ÓŠSO42-£¬
¹Ź“š°øĪŖ£ŗCu2+”¢NH4+£»SO42-£»
£Ø2£©°±Ęų±»50.00mL1.00mol/LŃĪĖįĶźČ«ĪüŹÕ£¬Ōņn£ØNH4+£©=n£ØNH3£©=n£ØHCl£©=0.05L”Į1mol/L=0.05mol£¬ÓɳĮµķÖŹĮææÉÖŖn£ØSO42-£©=n£ØBaSO4£©=$\frac{11.65g}{233g/mol}$=0.05mol£¬
ÓɵēŗÉŹŲŗćæÉÖŖ2n£ØCu2+£©+n£ØNH4+£©=2n£ØSO42-£©£¬n£ØCu2+£©=0.025mol£¬
Ōņn£ØH2O£©=$\frac{10g-0.05mol”Į18g/mol-0.025mol”Į64g/mol-0.05mol”Į96g/mol}{18g/mol}$=0.15mol£¬Ōņn£Ø£ØNH4£©2SO4£©£ŗn£ØCuSO4£©£ŗn£ØH2O£©=0.025£ŗ0.0525£ŗ0.15=1£ŗ1£ŗ6£¬Ōņ»ÆѧŹ½ĪŖ£ØNH4£©2SO4•CuSO4•6H2O»ņ£ØNH4£©2Cu£ØSO4£©2•6H2O£®
¹Ź“š°øĪŖ£ŗ£ØNH4£©2Cu£ØSO4£©2•6H2O»ņ£ØNH4£©2SO4•CuSO4•6H2O£®
µćĘĄ ±¾Ģāæ¼²éĪŽ»śĪļµÄĶʶĻ£¬ĪŖøßĘµæ¼µć£¬²ąÖŲӌѧɜµÄ·ÖĪöÄÜĮ¦”¢ŹµŃéÄÜĮ¦ŗĶ¼ĘĖćÄÜĮ¦µÄ漲飬½ā“šŹ±×¢Ņā“ÓµēŗÉŹŲŗćŗĶÖŹĮæŹŲŗćµÄ½Ē¶Č·ÖĪö£¬ÄѵĄÖŠµČ£®


 ĘŚÄ©100·Ö“³¹Ųŗ£µķæ¼ĶõĻµĮŠ“š°ø
ĘŚÄ©100·Ö“³¹Ųŗ£µķæ¼ĶõĻµĮŠ“š°ø Š”ѧÄÜĮ¦²āŹŌ¾ķĻµĮŠ“š°ø
Š”ѧÄÜĮ¦²āŹŌ¾ķĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
| ĪļÖŹ | A | B | C | D |
| ĘšŹ¼Ķ¶ĮĻ/mol | 2 | 2 | 3 | 0 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | NaOHČܽāŗóĪ“¾ĄäČ“£¬ŃøĖŁ×ŖŅʵ½ČŻĮæĘæÖŠ | |
| B£® | ČŻĮæĘæĪ“øÉŌļ | |
| C£® | ¶ØČŻŹ±ø©ŹÓŅŗĆę | |
| D£® | Ļ“µÓÉÕ±ŗĶ²£Į§°ōµÄČÜŅŗĪ“×ŖŅʵ½ČŻĮæĘæÖŠ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ¼õŠ”·“Ó¦ĪļÅØ¶Č£¬æɼõŠ”µ„Ī»Ģå»żÄŚ»ī»Æ·Ö×ӵİŁ·ÖŹż£¬“Ó¶ųŹ¹ÓŠŠ§Åöײ“ĪŹż¼õÉŁ | |
| B£® | ¶ŌÓŚÓŠĘųĢå²Ī¼ÓµÄ»Æѧ·“Ó¦£¬Čō¼õŠ”Ń¹Ēæ£Ø¼“Ą©“ó·“ӦȯĘ÷µÄĢå»ż£©£¬æɼõŠ”»ī»Æ·Ö×ӵİŁ·ÖŹż£¬“Ó¶ųŹ¹·“Ó¦ĖŁĀŹ¼õŠ” | |
| C£® | øıäĢõ¼žÄÜŹ¹»Æѧ·“Ó¦ĖŁĀŹŌö“óµÄÖ÷ŅŖŌŅņŹĒŌö“óĮĖ·“Ó¦Īļ·Ö×ÓÖŠ»ī»Æ·Ö×ÓµÄÓŠŠ§Åöײ¼øĀŹ | |
| D£® | ÄÜĮæøߵķÖ×ÓŅ»¶ØÄÜ·¢ÉśÓŠŠ§Åöײ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | µķ·ŪµÄ×ī¼ņŹ½£ŗCH2O | B£® | ĮŚōĒ»ł±½¼×ĖįµÄ½į¹¹¼ņŹ½£ŗ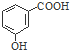 | ||
| C£® | 2-ŅŅ»ł-1£¬3-¶”¶žĻ©µÄ¼üĻߏ½£ŗ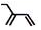 | D£® | ±½·Ö×ÓĒņ¹÷Ä£ŠĶ£ŗ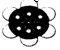 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
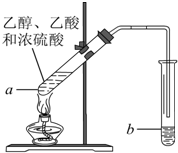 ŗģĘĻĢŃ¾ĘĆÜ·ā“¢“ꏱ¼äŌ½³¤£¬ÖŹĮæŌ½ŗĆ£¬ŌŅņÖ®Ņ»ŹĒ“¢“ę¹ż³ĢÖŠÉś³ÉĮĖÓŠĻćĪ¶µÄõ„£®ŌŚŹµŃéŹŅŅ²æÉŅŌÓĆČēĶ¼ĖłŹ¾µÄ×°ÖĆÖĘČ”ŅŅĖįŅŅõ„£¬Ēė»Ų“šĻĀĮŠĪŹĢā£®
ŗģĘĻĢŃ¾ĘĆÜ·ā“¢“ꏱ¼äŌ½³¤£¬ÖŹĮæŌ½ŗĆ£¬ŌŅņÖ®Ņ»ŹĒ“¢“ę¹ż³ĢÖŠÉś³ÉĮĖÓŠĻćĪ¶µÄõ„£®ŌŚŹµŃéŹŅŅ²æÉŅŌÓĆČēĶ¼ĖłŹ¾µÄ×°ÖĆÖĘČ”ŅŅĖįŅŅõ„£¬Ēė»Ų“šĻĀĮŠĪŹĢā£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
| N2+3H2?2NH3 | ||||
| ĪĀ¶Č | 25”ę | 200”ę | 400”ę | 600”ę |
| Ę½ŗā³£ŹżK | 5”Į108 | 650 | 0.507 | 0.01 |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com