
��9�֣����ڴ���ս��ʱ�ڣ��ҹ��Ϳ�ʼ������ʹ��������
��1��д����CO�ͳ�������Ҫ�ɷ�Fe2O3��Ϊԭ�������Ļ�ѧ����ʽ
��ʢˮ��������ˮ��Ӵ���һȦ�������⡣��������������� �������¼����ܽ�����ּӹ��������Ľ���˿�����������˲���ֵ� ������ĸ����
A����ʴ�� B����չ�� C�������� D��������
��2����ƴӷ�ˮ�л����������������ͭ��ʵ�鷽�����£�

����X�� ��
д������ʵ�鷽�����йط�Ӧ�Ļ�ѧ����ʽ
�� �� ��
��1��3CO+Fe2O3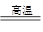 2Fe+3CO2
��ʪ����������ˮ�������Ӵ���
B
2Fe+3CO2
��ʪ����������ˮ�������Ӵ���
B
��2��Fe Fe+CuSO4=Cu+FeSO4 Fe+H2SO4=FeSO4+H2��
����������1��CO���л�ԭ�ԣ�������ұ������������ʽΪ3CO+Fe2O3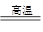 2Fe+3CO2
���ڳ�ʪ�Ļ����и�����������ʴ�����⡣�ӹ��������Ľ���˿���ǽ�������չ�ԣ���ѡB��
2Fe+3CO2
���ڳ�ʪ�Ļ����и�����������ʴ�����⡣�ӹ��������Ľ���˿���ǽ�������չ�ԣ���ѡB��
��2��Ҫ�õ�����ͭ����Ӧ���û���ͭ��ͬʱ�����������ʣ�����X����������ʽΪFe+CuSO4=Cu+FeSO4��ͭ��ϡ�����Ӧ���������ԣ�����Ҫ��ȥ����������������ϡ���ᣬ����ʽΪFe+H2SO4=FeSO4+H2����


 ͬ����ϰ���ϴ�ѧ������ϵ�д�
ͬ����ϰ���ϴ�ѧ������ϵ�д� ͬ����ϰ����ʦ����ѧ������ϵ�д�
ͬ����ϰ����ʦ����ѧ������ϵ�д� ����ϰ�⽭��ϵ�д�
����ϰ�⽭��ϵ�д� ѧ���쳵��������������������ϵ�д�
ѧ���쳵��������������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A.����������?
B.�ʵ������˺�̼��?
C.�����Ͻ�Ԫ��?
D.���Ʊ���Ľṹ����?
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ҹ��ڴ���ս��ʱ�ھͶ��ý��������ʻ����õ��൱�����ֵ�������������ı���������һ����Ҫ��ŷ�������ǧ�꣬��ô�����ʻ�������Ҫ������( )
A.����������
B.�ʵ������˺�̼��
C.�����Ͻ�Ԫ��
D.���Ʊ���Ľṹ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ҹ��ڴ���ս��ʱ�ھͶ��ý���������̼����2����������ȴ����õ��൱�����ֵ����������ı���������һ����Ҫ��ŷ�������ǧ�ꡣ��������ȴ�������Ҫ�����ǣ� ��
A.���������� B.�ʵ������˺�̼��
C.�����Ͻ�Ԫ�� D.���Ʊ���Ľṹ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�긣��ʡ����һ�и�һ��ѧ����ѧ���Ի�ѧ�Ծ����������� ���ͣ������
��9�֣����ڴ���ս��ʱ�ڣ��ҹ��Ϳ�ʼ������ʹ��������
��1��д����CO�ͳ�������Ҫ�ɷ�Fe2O3��Ϊԭ�������Ļ�ѧ����ʽ
��ʢˮ��������ˮ��Ӵ���һȦ�������⡣��������������� �������¼����ܽ�����ּӹ��������Ľ���˿�����������˲���ֵ� ������ĸ����
| A����ʴ�� | B����չ�� | C�������� | D�������� |

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com