
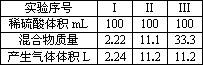
ŹµŃéŠņŗÅ | ¢ń | ¢ņ | ¢ó |
Ļ”ĮņĖįĢå»ż(mL) | 100 | 100 | 100 |
»ģŗĻĪļÖŹĮæ(g) | 2.22 | 11.1 | 33.3 |
²śÉśĘųĢåĢå»ż(L) | 2.24 | 11.2 | 11.2 |
(1)ŹµŃéÖŠ£¬ĮņĖį¹żĮæµÄŹµŃéĪŖ________”£(ĢīŠņŗÅ)
(2)Ļ”ĮņĖįµÄĪļÖŹµÄĮæÅضČĪŖ________”£
(3)¹ĢĢå·ŪÄ©ÖŠMgŗĶAlµÄĪļÖŹµÄĮæÖ®±ČĪŖ________”£
(4)µ±Ļ”ĮņĖįĢå»żĪŖ350mLŹ±£¬¼ÓČė33.3 gøĆ¹ĢĢå·ŪÄ©£¬³ä·Ö·“Ó¦ŗó²śÉśµÄĘųĢåĢå»ż(ÕŪĖć³É±ź×¼×“æö)ĪŖ_______________L”£

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ¼ŖĮÖŹ”³¤“ŗŹŠŹ®Ņ»ÖŠ2012½ģøßČżÉĻŃ§ĘŚĘŚÖŠæ¼ŹŌ»ÆѧŹŌĢā ĢāŠĶ£ŗ022
½«Ć¾·ŪŗĶĀĮ·Ū»ģŗĻ¾łŌČŗó£¬Č”²»Ķ¬ÖŹĮæµÄ»ģŗĻĪļ·Ö±š¼ÓČėµ½100 mLijÅØ¶ČµÄĻ”ĮņĖįÖŠ£¬²śÉśĘųĢåµÄĢå»ż(ŅŃÕŪŗĻ³É±ź×¼×“æö)Óė¼ÓČė»ģŗĻĪļµÄÖŹĮæ¹ŲĻµČēĻĀ±ķ£ŗ
(1)ŹµŃéÖŠ£¬ĮņĖį¹żĮæµÄŹµŃéĪŖ________(ĢīŠņŗÅ)£®
(2)Ļ”ĮņĖįµÄĪļÖŹµÄĮæÅضČĪŖ________£®
(3)¹ĢĢå·ŪÄ©ÖŠMgŗĶAlµÄĪļÖŹµÄĮæÖ®±ČĪŖ________£®
(4)µ±Ļ”ĮņĖįĢå»żĪŖ350 mLŹ±£¬¼ÓČė33.3æĖøĆ¹ĢĢå·ŪÄ©£¬³ä·Ö·“Ó¦ŗó²śÉśµÄĘųĢåĢå»ż(±ź×¼×“æöĻĀ)ĪŖ________L£®
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
A£®3 mol”¤L-1£¬HCl B£®4 mol”¤L-1£¬HNO3
C£®10 mol”¤L-1£¬NaOH D£®18 mol”¤L-1£¬H2SO4
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com