
��ҵ�������̿���Ҫ�ɷ�ΪMnO2���Ʊ�������صĹ���������ͼ��ʾ��
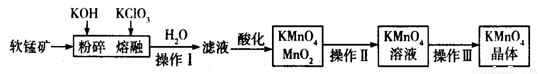
��1��������ص�������ǿ������Һ��������йأ��������������������Խ�ǿ������ ���������ữ���������Һ����____________������ţ���
A������ B��ϡ���� C������ D��������
��2������������Ʒֱ���______________��_______________��_______________��
��3�����̿����������KOH��KClO3������״̬�·�Ӧʱ����������__________________���ѧʽ������Ӧ���ˮ�ܽ�õ�����Һ����Ҫ����KCl��K2MnO4������Һ�ữʱ������Ӧ�����ӷ���ʽΪ _______________________________��
��4����֪KMnO4�����ȵľ������ữ��Na2C2O4��Ӧ����Mn2����CO2��ȡ�����Ƶõ�KMnO4��Ʒ0.33 g��ǡ����0.67 g Na2C2O4��ȫ��Ӧ����KMnO4�Ĵ���Ϊ________����
��10�֣�
��1��b (1��)
��2�� ���ˣ�1�֣� ���ˣ�1�֣� ������1�֣�
��3��KClO3��1�֣���3MnO42����4H��=2MnO4����MnO2����2H2O��2�֣�
��4��96��3�֣�
��������
 �����������1�������������������������ᣬ���᱾������ǿ�����ԣ���ѡ�����2�� �ɽ��в���������Һ���ʲ�����ӦΪ���ˣ�����MnO2������ˮ��������KMnO4��Һ���ʲ�����Ϊ���ˣ����в������ʹ��Һ����˾��壬�ʲ�����Ϊ��������3���ɲ��PԪ�صĻ��ϼۿ�֪������ӦΪKClO3����Һ����Ҫ�ɷ���K2MnO4���ữ��õ�KMnO4��MnO2���ʷ���ʽΪ��3MnO42����4H��=2MnO4����MnO2����2H2O����4�������⼰�����غ�ɵù�ϵʽΪ��
�����������1�������������������������ᣬ���᱾������ǿ�����ԣ���ѡ�����2�� �ɽ��в���������Һ���ʲ�����ӦΪ���ˣ�����MnO2������ˮ��������KMnO4��Һ���ʲ�����Ϊ���ˣ����в������ʹ��Һ����˾��壬�ʲ�����Ϊ��������3���ɲ��PԪ�صĻ��ϼۿ�֪������ӦΪKClO3����Һ����Ҫ�ɷ���K2MnO4���ữ��õ�KMnO4��MnO2���ʷ���ʽΪ��3MnO42����4H��=2MnO4����MnO2����2H2O����4�������⼰�����غ�ɵù�ϵʽΪ��
2KMnO4��5NaC2O4
2��158 5��134
m 0.67g
���m=0.316g
KMnO4�Ĵ���Ϊ0.316/0.33��100��=86��
���㣺����������ԭ��Ӧ�����㡢��ѧʵ�����������֪ʶ�㡣



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| 2 |
| 3 |
| 2 |
| 3 |
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��18�֣�����������̵���Ҫ������ͳ��õ��������������ǹ�ҵ�������̿���Ҫ�ɷ�MnO2���Ʊ�������ص�����ͼ��
��1�����̿�����Ŀ�� ��
���̿�KOH������ڿ����������ڷ�Ӧ����K2MnO4�Ļ�ѧ����ʽΪ��
�������������Ϊ ��
��2����Ӧ�ڵĻ�ѧ����ʽΪ ��
�������������п�ѭ�����õ�������Ca(OH)2��CO2�� �� ��
��ҵ��������ԭ������KMnO4�������ʽϵͣ��Ϻõ��Ʊ������ǵ�ⷨ����Pt��������Fe�����������K2MnO4��Һ�������ĵ缫��ӦʽΪ ��
��3��KMnO4��һ�ֽ��ȶ��Ļ�������չ��KMnO4��Һ�ķֽ��д����ã�����MnO2��KOH��O2���� MnO2Ҳ�Ǹ÷ֽⷴӦ��һ�ִ������������һ��ʵ�鷽������֤MnO2�Ը÷ֽⷴӦ���д��ԡ�����ʵ�������������ؽ��ۣ�
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��㶫ʡ������һ��ѧ������ѧ�����������۾�����ѧ���֣� ���ͣ��ʴ���
��18�֣�����������̵���Ҫ������ͳ��õ��������������ǹ�ҵ�������̿���Ҫ�ɷ�MnO2���Ʊ�������ص�����ͼ��
��1�����̿�����Ŀ�� ��
���̿�KOH������ڿ����������ڷ�Ӧ����K2MnO4�Ļ�ѧ����ʽΪ��
�������������Ϊ ��
��2����Ӧ�ڵĻ�ѧ����ʽΪ ��
�������������п�ѭ�����õ�������Ca(OH)2��CO2�� �� ��
��ҵ��������ԭ������KMnO4�������ʽϵͣ��Ϻõ��Ʊ������ǵ�ⷨ����Pt��������Fe�����������K2MnO4��Һ�������ĵ缫��ӦʽΪ ��
��3��KMnO4��һ�ֽ��ȶ��Ļ�������չ��KMnO4��Һ�ķֽ��д����ã�����MnO2��KOH��O2���� MnO2Ҳ�Ǹ÷ֽⷴӦ��һ�ִ������������һ��ʵ�鷽������֤MnO2�Ը÷ֽⷴӦ���д��ԡ�����ʵ�������������ؽ��ۣ�
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ��ɽ��ʡ�����и�����ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ������
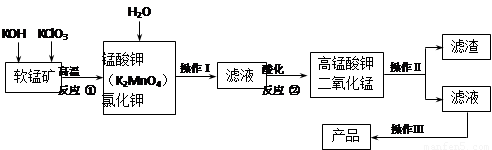
���������ʵ���Һ�ҽ�����зdz��㷺��Ӧ�á������ǹ�ҵ�������̿���Ҫ��MnO2���Ʊ�������ؾ����һ�ֹ������̣�

��1���������������У��ڢٲ���������Ϊ��д��ѧʽ�� ��
��2���ڢڲ���Ӧ�Ļ�ѧ����ʽ�� ��
��3����ĸҺ�Ƶ�KOHӦ����������� ��д����Ӧ��ѧ����ʽ ��
��4���������õ��������� ���������Ǹ���KMnO4��K2CO3���������� �ϵIJ��죬���������ᾧ�����ȹ��˵õ�KMnO4�־��塣
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014���Ĵ�ʡ�����и�����һ������Կ������ۻ�ѧ�Ծ��������棩 ���ͣ������
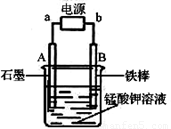
����������̵���Ҫ������ͳ�������������ʵ���Һ�ҽ�����зdz��㷺��Ӧ�á������ǹ�ҵ�������̿���Ҫ��MnO2���Ʊ�������ؾ����һ�ֹ������̣�

��1���������������_______��ʵ���ҽ��иò������õ��IJ��������� ��
��2����Ӧ�ڵIJ����У����������뻹ԭ��������ʵ� ��֮����______��
��3����Ӧ���С��ữ��ʱ����ѡ���������е�______������ĸ��ţ�
a��ϡ���� b��ϡ���� c��ϡ����
��4������������ҺҲ�����Ʊ�������أ���������ӦʽΪ��2H2O + 2e- = 2OH- + H2������ͼ��bΪ��Դ��_______�������ص�������Ӧʽ��_______�������������ռ���2.24 L H2����״��������õ��������______g������ʾ�������ӷŵ�˳��MnO42->OH-��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com