
 £¬ÄĘĄė×ÓŗĖĶāÄÜĮæ×īøߵĵē×ÓĪ»ÓŚ2p²ć£¬±Č½ĻĄė×Ó°ė¾¶“󊔣ŗr£ØNa+£©£¼r£ØO2-£©£ØĢī”°£¾”±£¬”°£¼”±»ņ”°=”±£©
£¬ÄĘĄė×ÓŗĖĶāÄÜĮæ×īøߵĵē×ÓĪ»ÓŚ2p²ć£¬±Č½ĻĄė×Ó°ė¾¶“󊔣ŗr£ØNa+£©£¼r£ØO2-£©£ØĢī”°£¾”±£¬”°£¼”±»ņ”°=”±£© £¬
£¬·ÖĪö £Ø1£©Ō×Ó½į¹¹Ź¾ŅāĶ¼ŹéŠ“£ŗŌ²ÄŚŹż×ÖĪŖŌ×ÓµÄÖŹ×ÓŹż£¬·ūŗűķŹ¾µēŠŌ£¬Ō²ĶāµÄ»”ĻßĪŖµē×Ó²ć£¬»”ĻßÉĻµÄŹż×ÖĪŖøĆ²ćÉĻµÄµē×ÓŹż£¬ĄėŌ²×ī½üµÄ»”Ļß±ķŹ¾µŚŅ»²ć£¬ŅĄ“ĪĻņĶāĪŖµŚ¶ž²ć”¢µŚČż²ćµČ£¬¾Ż“ĖŹéŠ“ŃõĄė×ӵĽį¹¹Ź¾ŅāĶ¼£»Ąė×ÓŗĖĶāÄÜĮæÓÉÄŚĻņĶāÄÜĮæÓɵĶµ½øߣ»¾ßÓŠĻąĶ¬µÄŗĖĶāµē×ÓÅŲ¼£¬Ō×ÓŠņŹżŌ½“󣬰ė¾¶Ō½Š”£»
£Ø2£©Ņ»°ćĄ“Ėµ£¬»īĘĆ½šŹōŗĶ»īĘĆ·Ē½šŹōŌŖĖŲÖ®¼äŅ׊Ī³ÉĄė×Ó¼ü£¬·Ē½šŹōŌŖĖŲÖ®¼äŅ׊Ī³É¹²¼Ū¼ü£¬µ„¼üÖŠÖ»ÓŠ¦Ņ¼ü£¬¾Ż“Ė·ÖĪö£»
£Ø3£©¶žŃõ»ÆĢ¼ĪŖ¹²¼Ū»ÆŗĻĪļ£¬Ęä·Ö×ÓÖŠŗ¬ÓŠĮ½øöĢ¼ŃõĖ«¼ü£»
£Ø4£©ĪļÖŹµÄĮæÓė·“Ó¦·Å³öµÄČČĮæ³ÉÕż±Č£¬øł¾ŻČČ»Æѧ·½³ĢŹ½µÄŹéŠ“·½·ØŹéŠ“£®
½ā“š ½ā£ŗ£Ø1£©OŌ×ÓµÄÖŹ×ÓŹżĪŖ8£¬ŃõĄė×ÓŗĖĶāČŻÄÉ10øöµē×Ó£¬ÓŠ2øöµē×Ó²ć£¬µŚŅ»µē×Ó²ćČŻÄÉ2øöµē×Ó£¬×īĶā²ćČŻÄÉ8øöµē×Ó£¬Ąė×Ó½į¹¹Ź¾ŅāĶ¼ĪŖ £¬ÄĘĄė×ÓŗĖĶāÅŲ¼ĪŖ£ŗ1s22s22p6£¬ÄÜĮæ×īøߵĵē×ÓĪ»ÓŚ2p£¬¾ßÓŠĻąĶ¬µÄŗĖĶāµē×ÓÅŲ¼£¬Ō×ÓŠņŹżŌ½“󣬰ė¾¶Ō½Š”£¬r£ØNa+£©£¼r£ØO2-£©£¬
£¬ÄĘĄė×ÓŗĖĶāÅŲ¼ĪŖ£ŗ1s22s22p6£¬ÄÜĮæ×īøߵĵē×ÓĪ»ÓŚ2p£¬¾ßÓŠĻąĶ¬µÄŗĖĶāµē×ÓÅŲ¼£¬Ō×ÓŠņŹżŌ½“󣬰ė¾¶Ō½Š”£¬r£ØNa+£©£¼r£ØO2-£©£¬
¹Ź“š°øĪŖ£ŗ £»2p£»£¼£»
£»2p£»£¼£»
£Ø2£©Ņ»°ćĄ“Ėµ£¬»īĘĆ½šŹōŗĶ»īĘĆ·Ē½šŹōŌŖĖŲÖ®¼äŅ׊Ī³ÉĄė×Ó¼ü£¬·Ē½šŹōŌŖĖŲÖ®¼äŅ׊Ī³É¹²¼Ū¼ü£¬¹żŃõ»ÆÄĘÖŠ¹żŃõøłĄė×ÓÓėÄĘĄė×ÓÖ®¼ä“ęŌŚĄė×Ó¼ü£¬Į½øöOŌ×Ó¼ä“ęŌŚ·Ē¼«ŠŌ¹²¼Ū¼ü£¬øĆ¹²¼Ū¼üĪŖµ„¼ü£¬Ö»ŗ¬ÓŠ¦Ņ¼ü£¬ĖłŅŌNa2O2¾§ĢåÖŠŗ¬ÓŠµÄ»Æѧ¼üÓŠĄė×Ó¼ü”¢¹²¼Ū¼ü£»
¹Ź“š°øĪŖ£ŗĄė×Ó¼ü”¢¹²¼Ū¼ü£»
£Ø3£©¶žŃõ»ÆĢ¼ÖŠ“ęŌŚĢ¼ŃõĖ«¼ü£¬Ģ¼Ō×ÓŗĶŃõŌ×Ó¶¼“ļµ½8µē×ÓĪČ¶Ø½į¹¹£¬¶žŃõ»ÆĢ¼µÄµē×ÓŹ½ĪŖ£ŗ £¬
£¬
¹Ź“š°øĪŖ£ŗ £»
£»
£Ø4£©16gFeŌŚĪļÖŹµÄĮæĪŖn=$\frac{m}{M}$=$\frac{16g}{56g/mol}$=$\frac{2}{7}$mol£¬ŌŚO2ÖŠĶźČ«Č¼ÉÕ£¬Éś³ÉŗŚÉ«¹ĢĢåĪŖĖÄŃõ»ÆČżĢś£¬·Å³ö112kJČČĮ棬æɵĆøĆ·“Ó¦µÄČČ»Æѧ·½³ĢŹ½£ŗ3Fe£Øs£©+2O2£Øg£©ØTFe3O4£Øs£©”÷H=-£Ø112”Į7”Į$\frac{3}{2}$£© kJ/mol=1176kJ/mol£¬
¹Ź“š°øĪŖ£ŗ3Fe£Øs£©+2O2£Øg£©ØTFe3O4£Øs£©”÷H=-1176kJ/mol£®
µćĘĄ ±¾Ģāæ¼²é½ĻĪŖ×ŪŗĻ£¬Éę¼°Ō×Ó½į¹¹Ź¾ŅāĶ¼ŹéŠ“”¢»Æѧ¼ü”¢µē×ÓŹ½”¢Ąė×Ó°ė¾¶“óŠ”±Č½Ļ”¢ČČ»Æѧ·½³ĢŹ½µÄŹéŠ“µČÖŖŹ¶£¬ĪŖøßæ¼µÄøßʵĢā£¬ĢāÄæÄѶČÖŠµČ£¬×¢ŅāÕĘĪÕµē×ÓŹ½”¢ČČ»Æѧ·½³ĢŹ½µÄŹéŠ“ŌŌņ£¬Ć÷Č·»Æѧ¼üµÄĄąŠĶ£®


 ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø
ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
| ŹµŃé | ѳʷµÄÖŹĮæ/g | NaOHČÜŅŗµÄĢå»ż/mL | °±ĘųµÄĢå»ż/L£Ø±ź×¼×“æö£© |
| 1 | 7.24 | 50.00 | 1.792 |
| 2 | 14.48 | 50.00 | 3.584 |
| 3 | 21.72 | 50.00 | 4.032 |
| 4 | 36.20 | 50.00 | 2.240 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Ė® | B£® | ¾Ę¾« | ||
| C£® | ĖįŠŌøßĆĢĖį¼ŲČÜŅŗ | D£® | ĒāŃõ»ÆÄĘČÜŅŗ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā

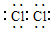 £¬MµÄѧ»ÆŹ½ĪŖNaClO£®
£¬MµÄѧ»ÆŹ½ĪŖNaClO£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 £¬Ēė»Ų“šĻĀĮŠĪŹĢā£®
£¬Ēė»Ų“šĻĀĮŠĪŹĢā£® £®
£®²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com