
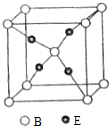
���� A��B��C��DΪ����ԭ��������������Ķ�����Ԫ�أ�A��E���������Ų�ʽ��Ϊns1����A����IA�壬EΪ�������ڸ���Ԫ�أ�E�ڲ�ȫ������EԪ��ԭ�Ӻ��������Ϊ2+8+18+1=29����EΪCu��B��Cͬ���壬��Ϊ���Ӱ��A2B�е����A2C������֪AΪHԪ�ء�BΪOԪ�ء�CΪSԪ�أ���D���ʵ�ˮ��Һ��ε�����ɫʯ����Һ����Һ�ȱ�����ɫ����DΪCl���ݴ˽��
��� �⣺A��B��C��DΪ����ԭ��������������Ķ�����Ԫ�أ�A��E���������Ų�ʽ��Ϊns1����A����IA�壬EΪ�������ڸ���Ԫ�أ�E�ڲ�ȫ������EԪ��ԭ�Ӻ��������Ϊ2+8+18+1=29����EΪCu��B��Cͬ���壬��Ϊ���Ӱ��A2B�е����A2C������֪AΪHԪ�ء�BΪOԪ�ء�CΪSԪ�أ���D���ʵ�ˮ��Һ��ε�����ɫʯ����Һ����Һ�ȱ�����ɫ����DΪCl��
��1��DΪCl����̬ԭ�ӵ����Ų�ʽΪ1s22s22p63s23p5���ʴ�Ϊ��1s22s22p63s23p5��
��2��H��O��S����Ԫ����SԪ�طǽ�������ǿ����Oԭ�ӵ縺����ʴ�Ϊ��O��
��3��SO3������Sԭ�Ӽ۲���Ӷ���Ϊ3+$\frac{6-2��3}{2}$=3��û�й¶Ե��ӣ�Ϊƽ���������Σ�������������������غϣ����ڷǼ��Է��ӣ��ʴ�Ϊ���Ǽ��ԣ�
��4��������Cuԭ����ĿΪ4��Oԭ����ĿΪ1+8��$\frac{1}{8}$=2��Cu��Oԭ����Ŀ֮��Ϊ2��1���ʸû����ﻯѧʽΪ��Cu2O��Bԭ������Χ4��Eԭ�����ڣ�����λ��Ϊ4��
�ʴ�Ϊ��Cu2O��4��
��5��һ�������£���Һ��S��Cl��Cu�ļ����ӻ�Ϻ������������[CuCl2]-����������CuԪ��Ϊ+1�ۣ�ͬʱ��Һ���ֻ�ɫ���ǣ���Ӧ�������ʣ��÷�Ӧ�����ӷ���ʽΪ��S2+Cl-+2Cu2+=2[CuCl2]-+S����
�ʴ�Ϊ��S2+Cl-+2Cu2+=2[CuCl2]-+S����
���� �����Ƕ����ʽṹ�Ŀ��飬�漰��������Ų��������ܡ����ӽṹ�����ʡ��������㡢�����ȣ���Ҫѧ���߱���ʵ�Ļ������Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
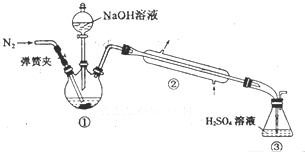 ijС���Է���м��ϡ���ᡢ���ͣ�NH4��2SO4��ҺΪԭ�ϣ�����һϵ�з�Ӧ�Ͳ����ϳ���dz����ɫ����X��Ϊȷ������ɣ���������ʵ�飮
ijС���Է���м��ϡ���ᡢ���ͣ�NH4��2SO4��ҺΪԭ�ϣ�����һϵ�з�Ӧ�Ͳ����ϳ���dz����ɫ����X��Ϊȷ������ɣ���������ʵ�飮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CO2�ĵ���ʽ�� | |
| B�� | Clԭ�ӵĽṹʾ��ͼ��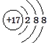 | |
| C�� | ������Ϊ53��������Ϊ78�ĵ�ԭ�ӵĺ��ط��ţ�${\;}_{53}^{131}$I | |
| D�� | 2��3-����-2-��ϩ�Ľṹ��ʽ��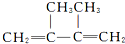 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 �õ�ⷨ�Ʊ����߷��Ӿۺ���--�ۺ��Ȼ�������ͼ��ʾ����һ�������½��У�����ܷ�
�õ�ⷨ�Ʊ����߷��Ӿۺ���--�ۺ��Ȼ�������ͼ��ʾ����һ�������½��У�����ܷ�| A�� | Cu�缫���Դ�������� | |
| B�� | �ۺ��Ȼ�����ѧʽ��x=4 | |
| C�� | ���ʱ�����ĵ缫��ӦʽΪ��2H++2e?�TH2�� | |
| D�� | ����Դ���ɵ����������缫�������γ�ԭ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ٵĻ�ѧ����ʽΪ��SiO2+C$\frac{\underline{\;����\;}}{\;}$Si+CO2�� | |
| B�� | ����٢ڢ���ÿ���ɻ�Ӧ1mol Si��ת��4mol���� | |
| C�� | ����������������ᷴӦ�����費��������ᷴӦ | |
| D�� | SiHCl3���е�33.0�棩�к���������SiCl4���е�67.6�棩��ͨ����������ᴿSiHCl3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NC13��N-C1��������CCl4��C-C1�������� | |
| B�� | NC13�����е�����ԭ�Ӿ��ﵽ8�����ȶ��ṹ | |
| C�� | NCl3�����Ǽ��Է��� | |
| D�� | NBr3�ķе��NCl3�ķе�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com