
| ЪЪСПЯЁЯѕЫс |
| Й§СПЬњЗл |
| зуСПЯЁСђЫс |
| Й§ТЫЁЂЯДЕг |
| ЫЎдЁ |
| ЫЎдЁ |
| ЫЎдЁ |



| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
| AЁЂ1.12LбѕЦј |
| BЁЂ1.12LЧтЦј |
| CЁЂ2.24LЧтЦј |
| DЁЂ2.24LбѕЦј |
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
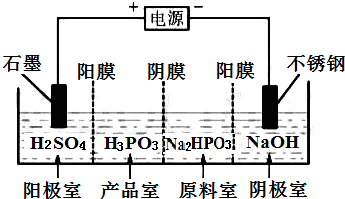 бЧСзЫсЃЈH3PO3ЃЉгызуСПЕФNaOHШмвКЗДгІЩњГЩNa2HPO3ЃЎ
бЧСзЫсЃЈH3PO3ЃЉгызуСПЕФNaOHШмвКЗДгІЩњГЩNa2HPO3ЃЎВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
AЁЂ
| ||
BЁЂ
| ||
| CЁЂ[H+]КЭ[OH-]ЕФГЫЛ§ | ||
| DЁЂOH-ЕФЮяжЪЕФСП |
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
 МзДМЦћгЭЪЧвЛжжаТФмдДЧхНрШМСЯЃЌПЩвдзїЮЊЦћгЭЕФЬцДњЮяЃЎЙЄвЕЩЯПЩгУCOКЭH2жЦШЁМзДМЃЌЛЏбЇЗНГЬЪНЮЊЃК
МзДМЦћгЭЪЧвЛжжаТФмдДЧхНрШМСЯЃЌПЩвдзїЮЊЦћгЭЕФЬцДњЮяЃЎЙЄвЕЩЯПЩгУCOКЭH2жЦШЁМзДМЃЌЛЏбЇЗНГЬЪНЮЊЃК| c(CO)Ц№ЪМ |
| c(H2)Ц№ЪМ |
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
| AЁЂФЦ | BЁЂЭ | CЁЂТС | DЁЂЬњ |
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
 ОнБЈЕРЃЌдквЛЖЈЬѕМўЯТЃЌN2дкВєгаЩйСПбѕЛЏЬњЕФЖўбѕЛЏюбДпЛЏМСБэУцФмгыЫЎЗЂЩњЗДгІЃЌжївЊВњЮяЮЊNH3ЃЌЯргІЕФЗДгІЗНГЬЪНЮЊЃК2N2ЃЈgЃЉ+6H2O ЃЈgЃЉ?4NH3ЃЈgЃЉ+3O2ЃЈgЃЉЁїH=Q kJ/mol
ОнБЈЕРЃЌдквЛЖЈЬѕМўЯТЃЌN2дкВєгаЩйСПбѕЛЏЬњЕФЖўбѕЛЏюбДпЛЏМСБэУцФмгыЫЎЗЂЩњЗДгІЃЌжївЊВњЮяЮЊNH3ЃЌЯргІЕФЗДгІЗНГЬЪНЮЊЃК2N2ЃЈgЃЉ+6H2O ЃЈgЃЉ?4NH3ЃЈgЃЉ+3O2ЃЈgЃЉЁїH=Q kJ/molВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
| 10Ёц | 20Ёц | 30Ёц | 40Ёц | 50Ёц | 60Ёц | |
| KNO3 | 20.9 | 31.6 | 45.8 | 63.9 | 85.5 | 110.0 |
| KCl | 31.0 | 34.0 | 37.0 | 40.0 | 42.0 | 45.5 |

ВщПДД№АИКЭНтЮі>>
АйЖШжТаХ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com