
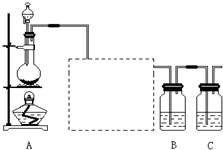
ĪŖČ·ČĻĖįŠŌĒæČõ£ŗHCL£¾H2CO3£¾H2SiO3£¬Ä³Ń§ÉśÉč¼ĘĮĖČēĻĀĶ¼ĖłŹ¾µÄ×°ÖĆ£¬ĶعżŅ»“ĪŹµŃ鼓æÉ“ļµ½ÄæµÄ”£

Ēė»Ų“šĻĀĮŠĪŹĢā:
£Ø1£©×¶ŠĪĘæ֊װӊijæÉČÜŠŌÕżŃĪČÜŅŗ£¬øĆŃĪŌŚ·ÖĄąÉĻŹōÓŚ £ØĢī”°Ģ¼ĖįŃĪ”±”¢”°ĮņĖįŃĪ”±»ņ”°¹čĖįŃĪ”±£©”£
£Ø2£©×°ÖĆBÖŠĖłŹ¢µÄŹŌ¼ĮĪŖ±„ŗĶNaHCO3ČÜŅŗ£¬Ęä×÷ÓĆŹĒ ”£
£Ø3£©×°ÖĆCÖŠĖłŹ¢µÄŹŌ¼ĮŹĒ £ØĢī”°NaCl”±”¢”°Na2CO3”±»ņ”°Na2SiO3”±£©ČÜŅŗ£¬CÖŠ·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒ ”£
 ¼¤»īĖ¼Ī¬ÓżÓæĪĢĆĻµĮŠ“š°ø
¼¤»īĖ¼Ī¬ÓżÓæĪĢĆĻµĮŠ“š°ø »īĮ¦ŹŌ¾ķĻµĮŠ“š°ø
»īĮ¦ŹŌ¾ķĻµĮŠ“š°ø æĪæĪÓÅÄÜĮ¦ÅąÓÅ100·ÖĻµĮŠ“š°ø
æĪæĪÓÅÄÜĮ¦ÅąÓÅ100·ÖĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
 ”ś
”ś -CH2COOH+HCl
-CH2COOH+HCl ”ś
”ś -CH2COOH+HCl
-CH2COOH+HCl| Ā±“śĖį | F3CCOOH | Cl3CCOOH | F2CHCOOH | FCH2COOH | CH3CH2CHClCOOH |
| pKa | 0.23 | 0.65 | 1.24 | 2.66 | 2.85 |
| Ā±“śĖį | ClCH2COOH | BrCH2COOH | ICH2COOH | CH3CHClCH2COOH | Cl¢ŪCH2CH2CH2COOH |
| pKa | 2.86 | 2.90 | 3.18 | 4.06 | 4.52 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
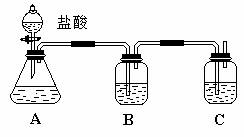
 ŅŃÖŖ·Ē½šŹōµ„ÖŹĮņ£ØS£©ŹĒµ»ĘÉ«¹ĢĢå·ŪÄ©£¬ÄŃČÜÓŚĖ®£®ĪŖĮĖŃéÖ¤ĀČŌŖĖŲµÄ·Ē½šŹōŠŌ±ČĮņŌŖĖŲµÄ·Ē½šŹōŠŌĒ棬ij»ÆѧŹµŃ銔×éÉč¼ĘĮĖČēĻĀŹµŃ飬Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
ŅŃÖŖ·Ē½šŹōµ„ÖŹĮņ£ØS£©ŹĒµ»ĘÉ«¹ĢĢå·ŪÄ©£¬ÄŃČÜÓŚĖ®£®ĪŖĮĖŃéÖ¤ĀČŌŖĖŲµÄ·Ē½šŹōŠŌ±ČĮņŌŖĖŲµÄ·Ē½šŹōŠŌĒ棬ij»ÆѧŹµŃ銔×éÉč¼ĘĮĖČēĻĀŹµŃ飬Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012-2013ѧğ¹ć¶«Ź”·šÉ½ŹŠÄĻŗ£ĒųøßČżæ¼Ē°³å“ĢĄķ×Ū»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗ¼ĘĖćĢā
ĻÖÓŠ±½¼×Ėį”¢±½·ÓČÜÓŚŅŅ“¼ĖłµĆµÄ»ģŗĻŅŗ£¬Ä³Ķ¬Ń§Éč¼Ę·½°ø·ÖĄėČżÖÖĪļÖŹ£¬²¢¼ģŃéĘäÖŠµÄijŠ©Ąė×ÓŗĶĪļÖŹ”£
ŅŃÖŖ£ŗ£Ø1£©ĖįŠŌĒæČõ£ŗHCl>±½¼×Ėį>H2CO3>±½·Ó> HCO3£ £Ø2£©²æ·ÖĪļĄķ²ĪŹżČēĻĀ£ŗ
|
|
ĆÜ¶Č £Øg”¤mL£1£© |
ČŪµć £Ø”ę£© |
·Šµć £Ø”ę£© |
ČܽāŠŌ |
|
±½¼×Ėį
|
1£®2659 |
122£®13”ę |
249”ę |
Ī¢ČÜÓŚĖ®£¬Ņ×ČÜÓŚŅŅ“¼”¢ŅŅĆѵČÓŠ»śČܼĮ |
|
±½·Ó
|
1£®07
|
40£®6
|
181£®9
|
Ņ×ČÜÓŚŅŅ“¼”¢ŅŅĆŃ£¬65”ęŅŌÉĻÄÜÓėĖ®»„ČÜ |
|
ŅŅ“¼ |
0£®79 |
-114£®3 ”ćC |
78£®5 |
ÓėĖ®»ģČÜ£¬æÉ»ģČÜÓŚĆŃ”¢ĀČ·Ā”¢øŹÓĶµČ¶ąŹżÓŠ»śČܼĮ |
¹©Ń”ŌńŹŌ¼Į£ŗ10%ĒāŃõ»ÆÄĘČÜŅŗ”¢0.1mol/LŃĪĖį”¢0.1mol/LNa2CO3”¢0.1mol/L NaHCO3”¢ÅØäåĖ®”¢ÉśŹÆ»Ņ”¢0.1mol/L FeCl3”¢0.1mol/L BaCl2”¢CO2”¢0.1mol/LäåĖ®”¢³ĪĒåŹÆ»ŅĖ®
£Ø1£©·ÖĄėĪļÖŹĮ÷³ĢČēĻĀ£ŗ

¢ŁĪļÖŹCŹĒ_____________£¬²Ł×÷IVŹĒ_____________”£
¢Ś²Ł×÷III·¢ÉśµÄÖ÷ŅŖ»Æѧ·“Ó¦·½³ĢŹ½_________________ _______________________________”£
¢Ū»ģŗĻŅŗ2ÖŠ¼ÓČėÉśŹÆ»ŅµÄŌŅņŹĒ_______”£
£Ø2£©øĆĶ¬Ń§¼ģŃé»ģŗĻŅŗ1ÖŠŹĒ·ńŗ¬ÓŠ±½·ÓŗĶNaHCO3£¬ŅŌÖ¤Ć÷ĖįŠŌµÄĒæČõ”£
|
ŹµŃé²½Öč |
ĻÖĻóŗĶ½įĀŪ |
|
¢ŁČ”ÉŁĮæ»ģŗĻŅŗ1ÓŚŹŌ¹Ü£¬µĪ¼Ó________________________£¬ Õńµ“£¬¾²ÖĆ |
|
|
¢ŚČ”ÉŁĮæ²½Öč¢ŁÉĻ²ćĒåŅ¹ÓŚŹŌ¹Ü£¬µĪ¼Ó__________________ ____________________________________________________ |
³ĪĒåŹÆ»ŅĖ®±ä»ė×Ē ĖµĆ÷»ģŗĻŅŗ1ŗ¬ÓŠHCO3£ |
|
¢ŪĮķȔɣĮæ»ģŗĻŅŗ1ÓŚŹŌ¹Ü£¬µĪ¼Ó___________________£¬Õńµ“ |
_________________________£¬ ĖµĆ÷»ģŗĻŅŗ1²»ŗ¬ÓŠ·ÓōĒ»ł |
£Ø3£©³ĘČ”2.0g±½¼×ĖįŗĶ±½·ÓµÄ»ģŗĻ¹ĢĢåČÜÓŚ×ćĮæŅŅ“¼ÖŠ£¬µĪ¼Ó×ćĮ汄ŗĶNaHCO3ČÜŅŗ£¬²āµĆ·Å³öµÄCO2£Ø±ź×¼×“æöĻĀ£¬²»æ¼ĀĒCO2ČÜÓŚĖ®£©ĪŖ33.6mL £¬Ōņ±½¼×ĖįµÄÖŹĮæ·ÖŹżĪŖ_________________________£ØÖ»ĮŠŹ½£¬²»¼ĘĖć£©£¬½į¹ūĪŖ________”££Ø½į¹ū±£Įō1Ī»Š”Źż£©£Ø±½¼×ĖįµÄĻą¶Ō·Ö×ÓÖŹĮæĪŖ122£¬±½·ÓĻą¶Ō·Ö×ÓÖŹĮæĪŖ94£©
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĪŖČ·ČĻĻĀĆęČżÖÖĖįµÄĖįŠŌĒæČõ£ŗHCl£¾H2CO3£¾H2SiO3£¬ĻėĻėĶ¬Ń§Éč¼ĘĮĖČēĶ¼ĖłŹ¾µÄ×°ÖĆ£¬ĶعżŅ»“ĪŹµŃ鼓æÉ“ļµ½ÄæµÄ”£
 Ēė»Ų“šĻĀĮŠĪŹĢā:
Ēė»Ų“šĻĀĮŠĪŹĢā:
£Ø1£©×¶ŠĪĘæ֊װӊijæÉČÜŠŌÕżŃĪČÜŅŗ£¬øĆŃĪŌŚ·ÖĄąÉĻŹōÓŚ”””””””””””””” ”””””””” £ØĢī”°Ģ¼ĖįŃĪ”±”¢”°ĮņĖįŃĪ”±»ņ”°¹čĖįŃĪ”±£©”£
£Ø2£©×°ÖĆBÖŠĖłŹ¢µÄŹŌ¼ĮĪŖ±„ŗĶNaHCO3ČÜŅŗ£¬Ęä×÷ÓĆŹĒ”””””””””””””””””””””””””” ”£
£Ø3£©×°ÖĆCÖŠĖłŹ¢µÄŹŌ¼ĮŹĒ”””””””””””””””” £ØĢī”°NaCl”±”¢”°Na2CO3”±»ņ”°Na2SiO3”±£©ČÜŅŗ£¬CÖŠ·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒ”””””””””””””””””””””””””””””””””””””””””””””” ”””£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com