
Ϊ��֤���Ҵ������к�����ԭ�ӣ��ֲ���һ��װ�ý���ʵ�飬�Ը�������װ���е��Լ���ʵ�����ش��й����⣺

��1��װ������װ���Լ�����Aƿװ��ˮ�Ҵ����ڷ���ˮ��X��
��B�������װ��ʯ�ң�
��C��D�ж�װŨH2SO4��
��Eƿװ�Լ�Y��
��2��ʵ������������ˮԡ����Aƿ����D��ŨH2SO4��������E�����Լ�Y���ã�����C�е����д������ݷų���Aƿ��X��ɫ����B�лӷ���������ɵ�ȼ��
�ش��������⣺
��1��Eƿ����װ���Լ�Y��____________��
�ٱ���ʳ��ˮ ��MnO2��NaCl�Ļ���� ��ŨHCl
��2��D��ŨH2SO4�����������_________________��C��ŨH2SO4�����������__________��
��3��Aƿ�з�����Ӧ�Ļ�ѧ����ʽ��____________________����Ӧ������___________�������ɵ�_________��д���ƣ���B���ڴ���ȼ��
��4����ˮ��X��ѡ��__________��������ָʾ�����õ�ԭ����____________________��
��5����ʵ����֤���Ҵ������к�����ԭ�ӵ�������____________________��
��6�������װ���е�Cƿȥ���ܷ�ó����ս��ۣ�____________________��Ϊʲô��
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| �� |
| ||
| �� |
| ����ø |
| �ƻ�ø |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��������ѧ���¿γ̡���ѧ2����(³�ư�) ���ͣ�058
ijУ��ѧ������ȤС���ѧ��Ϊ����֤�Ҵ��ķ��ӽṹ���������ʵ������Ҵ������Ԫ�صIJⶨ������ʽ�IJⶨ�����ӽṹ�IJⶨ��

(1)���Ǿ�����ȼ���Ҵ�����������ȷ���Ҵ��к���C��H����Ԫ�أ���Ҫ˵�����ǵľ��������
��֤��������Ԫ�صIJ�����___________��
��֤������̼Ԫ�صIJ�����___________��
(2)Ҫ��ȼ�շ�������֤ʵ�Ҵ��л�������Ԫ��ʱ����ȡ��һЩʵ�����ݣ���Щ����Ӧ����________��
(3)Ϊȷ���Ҵ��ķ���ʽ����(2)�������⣬���費��Ҫ�ⶨ�Ҵ�����Է���������_______________________��
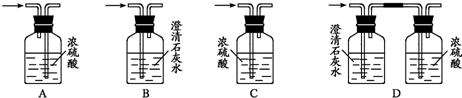
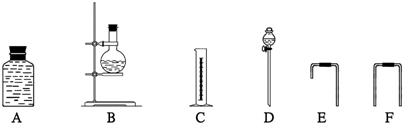
(4)Ϊ�ⶨ�Ҵ����ӽṹ������������ˮ�Ҵ��ͽ����Ʒ�Ӧ�ռ����������ķ�����ѡ��������ͼ��ʾ������(�е���������˫����Ƥ��)��
��װ�õ�����˳����________��________��________��________��________��________��
����ʵ��֤���Ҵ����ӵĽṹ��ʽ��CH3CH2OH������CH3OCH3��������_______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��10�֣�ijУ��ѧ����С���ѧ��Ϊ����֤�Ҵ��ķ��ӽṹ���������ʵ������Ҵ��ľ��ơ��Ҵ����Ԫ�صIJⶨ������ʽ�IJⶨ�����ӽṹ�IJⶨ��
��1���������Ҵ��к�����ˮ������ֱ������ķ���������Ч��ȥˮ��ͨ�������м��� ��Ȼ������֤���Ҵ����ٺ�ˮ������һ���Լ����飬�����Լ��� ��
��2����ȼ���Ҵ���������ķ���ȷ������C��H��O����Ԫ�ء�
a��֤������HԪ�صIJ����� ��
b��֤������OԪ��ʱ����ȡ�õ�ʵ�������ǣ�
CO2��������H2O�������� ��
��3��Ϊȷ���Ҵ��ķ���ʽ����ͨ��(2)��ȡ�Ҵ���ʵ��ʽ֮���Ƿ�����ٲⶨ�Ҵ�����Է�����������ȷ�������ʽ (���ǡ���)�������� ��
��4���ⶨ�Ҵ��ķ��ӽṹ
a�������ú�������Dzⶨ����ͨ�����ú���������� ���������շ壬����ȷ���Ҵ��Ľṹ��CH3CH2OH������CH3OCH3��(�C��H������C��C������C��O������O��H��)
b�������ú˴Ź����Dzⶨ���������Ҵ��ĺ˴Ź���������Ӧ�� �����շ塣
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�켪��ʡ�߶���ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
ijУ��ѧ������ȤС���ѧ��Ϊ����֤�Ҵ��ķ��ӽṹ���������ʵ������Ҵ����Ԫ�صIJⶨ������ʽ�IJⶨ�����ӽṹ�IJⶨ��
��1�����Ǿ�����ȼ���Ҵ�����������ȷ���Ҵ��к���C��H����Ԫ�ء���Ҫ˵�����ǵľ����������֤��������Ԫ�صIJ�����________________________________________________________ _
________________________________________________________________________________________��
��֤������̼Ԫ�صIJ�����________________________________________________
_______________________________________________________________________
��2��Ҫ��ȼ�շ�������֤ʵ�Ҵ��л�������Ԫ��ʱ����ȡ��һЩʵ�����ݣ���Щ����Ӧ����________________________��
��3��Ϊȷ���Ҵ��ķ���ʽ������2���������⣬���費��Ҫ�ⶨ�Ҵ�����Է�������?
_______________________________________________________________________
��4��Ϊ�ⶨ�Ҵ����ӽṹ������������ˮ�Ҵ��ͽ����Ʒ�Ӧ�ռ����������ķ�����ѡ��������ͼ��ʾ������(�е���������˫����Ƥ��)��

��װ�õ�����˳����______��______��______��______��_______��_______��
����֪��ˮ�ƾ����ܶ�Ϊ0��789 g��cmһ3����ȡ2��0mL�ƾ�����Ӧ��ȫ��(�ƹ���)���ռ�390 mL���塣���Ҵ��������ܱ���ȡ��������ԭ����Ϊ_______���ɴ˿�ȷ���Ҵ��ĽṹΪ______________________������______________��
��5��ʵ�����ⶨ�Ľ��ƫ�ߣ����������ԭ����(��д���)��______________
A����ʵ���������½���
B����ˮ�ƾ��л������״�
C����ˮ�ƾ����Ʒ�Ӧ������ȫ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013������ʡ�߶��������¿���ѧ�Ծ� ���ͣ�ʵ����
��10�֣�ijУ��ѧ����С���ѧ��Ϊ����֤�Ҵ��ķ��ӽṹ���������ʵ������Ҵ��ľ��ơ��Ҵ����Ԫ�صIJⶨ������ʽ�IJⶨ�����ӽṹ�IJⶨ��
��1���������Ҵ��к�����ˮ������ֱ������ķ���������Ч��ȥˮ��ͨ�������м��� ��Ȼ������֤���Ҵ����ٺ�ˮ������һ���Լ����飬�����Լ��� ��
��2����ȼ���Ҵ���������ķ���ȷ������C��H��O����Ԫ�ء�
a��֤������HԪ�صIJ����� ��
b��֤������OԪ��ʱ����ȡ�õ�ʵ�������ǣ�
CO2��������H2O�������� ��
��3��Ϊȷ���Ҵ��ķ���ʽ����ͨ��(2)��ȡ�Ҵ���ʵ��ʽ֮���Ƿ�����ٲⶨ�Ҵ�����Է�����������ȷ�������ʽ (���ǡ���)�������� ��
��4���ⶨ�Ҵ��ķ��ӽṹ
a�������ú�������Dzⶨ����ͨ�����ú���������� ���������շ壬����ȷ���Ҵ��Ľṹ��CH3CH2OH������CH3OCH3��(�C��H������C��C������C��O������O��H��)
b�������ú˴Ź����Dzⶨ���������Ҵ��ĺ˴Ź���������Ӧ�� �����շ塣
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com