
ĻÖÓŠA”¢B”¢C”¢D”¢E”¢FĮłÖÖ³£¼ū»ÆŗĻĪļ£¬ŅŃÖŖĖüĆĒ°üŗ¬µÄŃōĄė×ÓÓŠK£«”¢Ag£«”¢Na£«”¢
Ba2£«”¢Fe2£«”¢Al3£«£¬ŅõĄė×ÓÓŠCl£”¢OH£”¢AlO2-”¢NO3-”¢SO42-”¢CO32-”£½«ĖüĆĒ·Ö±šÅä³É0.1 mol/LµÄČÜŅŗ½ųŠŠČēĻĀŹµŃé£ŗ
¢Ł²āµĆČÜŅŗA”¢C”¢E¾ł³Ź¼īŠŌ£¬ĒŅ¼īŠŌA£¾E£¾C£¬EµÄŃęÉ«³ŹĒ³×ĻÉ«£ØĶø¹żĄ¶É«īܲ£Į§¹Ū²ģ£©£»¢ŚĻņBČÜŅŗÖŠµĪ¼ÓĻ”°±Ė®ÖĮ¹żĮ棬ĻČÉś³É³Įµķ£¬ŗó³ĮµķČ«²æČܽā£»¢ŪĻņFČÜŅŗÖŠµĪ¼ÓĻ”ĻõĖį£¬ČÜŅŗ±ä³É×Ų»ĘÉ«£¬ĒŅÓŠĪŽÉ«ĘųĢåÉś³É£»¢ÜĻņDČÜŅŗÖŠµĪ¼ÓBa£ØNO3£©2ČÜŅŗĪŽĆ÷ĻŌĻÖĻó”£
£Ø1£©Š“³öA”¢D”¢E”¢FµÄ»ÆѧŹ½£ŗ
A________£»D________£»E________£»F________”£
£Ø2£©ÓĆĄė×Ó·½³ĢŹ½½āŹĶCČÜŅŗ³Ź¼īŠŌµÄŌŅņ£ŗ__________________________________”£
£Ø3£©Š“³öŹµŃé¢ŪÖŠ·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ________________________________”£
 Ē§ĄļĀķ×ßĻņ¼ŁĘŚĘŚÄ©·ĀÕęŹŌ¾ķŗ®¼ŁĻµĮŠ“š°ø
Ē§ĄļĀķ×ßĻņ¼ŁĘŚĘŚÄ©·ĀÕęŹŌ¾ķŗ®¼ŁĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
(17·Ö)Ģś¼°Ęä»ÆŗĻĪļŌŚČÕ³£Éś»ī”¢Éś²śÖŠÓ¦ÓĆ¹ć·ŗ”£ĀČ»ÆĢśŗĶøßĢśĖį¼Ų¶¼ŹĒ³£¼ūµÄĖ®“¦Ąķ¼Į”£ĻĀĶ¼ĪŖÖʱøĀČ»ÆĢś¼°½ųŅ»²½Ńõ»ÆÖʱøøßĢśĖį¼ŲµÄ¹¤ŅÕĮ÷³Ģ”£
Ēė»Ų“šĻĀĮŠĪŹĢā
£Ø1£©ŅŃÖŖ£ŗ¢ŁFe2O3£Øs£©+3C(ŹÆÄ«) 2Fe(s)+3CO£Øg£©”÷H=+489.0KJ”¤mol
2Fe(s)+3CO£Øg£©”÷H=+489.0KJ”¤mol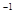 £»
£»
¢ŚC(ŹÆÄ«)+CO2£Øg£© 2CO(g)”÷H=+172.5KJ”¤mol
2CO(g)”÷H=+172.5KJ”¤mol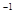 £»ÓĆ³ąĢśæóĪŖŌĮĻŌŚøßĀÆĮ¶Ģś¹ż³ĢÖŠ·¢ÉśµÄÖ÷ŅŖ·“Ó¦ĪŖFe2O3£Øs£©+3CO(g)
£»ÓĆ³ąĢśæóĪŖŌĮĻŌŚøßĀÆĮ¶Ģś¹ż³ĢÖŠ·¢ÉśµÄÖ÷ŅŖ·“Ó¦ĪŖFe2O3£Øs£©+3CO(g) 2Fe(s)+3CO2£Øg£©”÷H= KJ”¤mol
2Fe(s)+3CO2£Øg£©”÷H= KJ”¤mol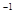 ”£
ӣ
£Ø2£©ĪüŹÕ¼ĮxµÄČÜÖŹĪŖ____________(Š“»ÆѧŹ½)”£
£Ø3£©Ńõ»Æ¼ĮYĪŖ”°84Ļū¶¾Ņŗ”±µÄÓŠŠ§³É·Ö£¬ŌņŌŚ¼īŠŌĢõ¼žĻĀ·“Ó¦¢ŁµÄĄė×Ó·½³ĢŹ½ĪŖ
__________________________________________________________________
£Ø4£©¹ż³Ģ¢ŚŹĒŌŚÄ³µĶĪĀĻĀ½ųŠŠµÄ£¬·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ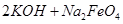 =
=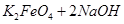 £¬ĖµĆ÷“ĖĪĀ¶ČĻĀ
£¬ĖµĆ÷“ĖĪĀ¶ČĻĀ __________
__________ 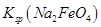 (Ģī”°>”±»ņ”°<”±)”£
(Ģī”°>”±»ņ”°<”±)”£
¼Ł¶Ø“Ė¹ż³ĢÖŠ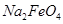 ĶźČ«×Ŗ»ÆĪŖ
ĶźČ«×Ŗ»ÆĪŖ £¬Čō×īÖÕÖĘµĆ“Ö²śĘ·
£¬Čō×īÖÕÖĘµĆ“Ö²śĘ· 206.25t£¬²śĘ·“æ¶ČĪŖ96£„£¬ŌņĄķĀŪÉĻÖĮÉŁŠčŅŖŃõ»Æ¼ĮYµÄÖŹĮæŹĒ___________t”£
206.25t£¬²śĘ·“æ¶ČĪŖ96£„£¬ŌņĄķĀŪÉĻÖĮÉŁŠčŅŖŃõ»Æ¼ĮYµÄÖŹĮæŹĒ___________t”£
£Ø5£©øßĢśµē³ŲŹĒŅ»ÖÖŠĀŠĶ¶ž“Īµē³Ų£¬µē½āŅŗĪŖĒæ¼īČÜŅŗ£¬Ęäµē³Ų·“Ó¦ĪŖ£ŗ3Zn+2K2FeO4+8H2O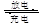 3Zn£ØOH£©2+2Fe£ØOH£©3+4KOH£¬·ÅµēŹ±µē³ŲµÄøŗ¼«·“Ó¦Ź½ĪŖ______________________________________”£
3Zn£ØOH£©2+2Fe£ØOH£©3+4KOH£¬·ÅµēŹ±µē³ŲµÄøŗ¼«·“Ó¦Ź½ĪŖ______________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ijŠ£Ń§Éś»ÆѧŹµŃ銔×é,ĪŖŃéÖ¤·Ē½šŹōŌŖĖŲĀȵÄŃõ»ÆŠŌĒæÓŚĮņŗĶµŖ,Éč¼ĘĮĖŅ»Ģ׏µŃé×°ÖĆ(²æ·Ö¼Š³Ö×°ÖĆŅŃĀŌČ„):
(1)Š“³öAÖŠ·“Ó¦µÄĄė×Ó·½³ĢŹ½”””””””””””£
(2)BÖŠ³öĻÖ»ĘÉ«»ė×ĒĻÖĻó,²śÉś“ĖĻÖĻóµÄĄė×Ó·½³ĢŹ½”””””””””””””””””””””””””””””£
(3)DÖŠøÉŌļ¹ÜÖŠ³öĻÖµÄĻÖĻóŹĒ £¬»Æѧ·½³ĢŹ½”””””””””””””””””””””””£
(4)ÓŠĶ¬Ń§ČĻĪŖDÖŠµÄĻÖĻó²¢²»ÄÜĖµĆ÷ĀȵÄŃõ»ÆŠŌ“óÓŚµŖ,ŠčŅŖŌŚCÖ®Ē°¼Ó×°Ļ“Ęų×°ÖĆ£¬ĒėÖø³öĻ“Ęų×°ÖĆŹ¢×°ŹŌ¼Į”””””””””””””£
(5)»¹ÓŠŹ²Ć“·½·ØÄÜÖ¤Ć÷Ńõ»ÆŠŌCl2>S ,ÓĆŅ»ÖÖĻą¹ŲŹĀŹµĖµĆ÷”””””””””””””””””””””£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ÓɼøÖÖĄė×Ó»ÆŗĻĪļ×é³ÉµÄ»ģŗĻĪļ£¬ŗ¬ÓŠŅŌĻĀĄė×ÓÖŠµÄČōøÉÖÖ£ŗK£«”¢NH”¢Ba2£«”¢Al3£«”¢Fe3£«”¢Cl£”¢SO42-”¢CO32-£¬½«øĆ»ģŗĻĪļČÜÓŚĖ®ŗóµĆ³ĪĒåČÜŅŗ£¬ĻÖČ”3·Ż100 mLøĆČÜŅŗ·Ö±š½ųŠŠČēĻĀŹµŃé£ŗ
| ŠņŗÅ | ŹµŃéÄŚČŻ | ŹµŃé½į¹ū |
| 1 | ¼Ó¹żĮæŃĪĖį | ĪŽĘųĢå²śÉś |
| 2 | ¼Ó×ćĮæNaOHČÜŅŗ²¢¼ÓČČ | ŹÕ¼Æµ½ĘųĢå1.12 L(ŅŃÕŪĖć³É±ź×¼×“æöĻĀµÄĢå»ż)£¬²¢ÓŠŗģŗÖÉ«³ĮµķÉś³É£¬½«³Įµķ¹żĀĖĻ“µÓ”¢øÉŌļ”¢×ĘÉÕ£¬³ĘÖŲµĆ1.60 g¹ĢĢ唣 |
| 3 | ¼Ó×ćĮæBaCl2ČÜŅŗŹ±£¬¶ŌĖłµĆ³Įµķ½ųŠŠĻ“µÓ”¢øÉŌļ”¢³ĘĮæ | µŚŅ»“Ī³ĘĮæ¶ĮŹżĪŖ2.33 g |
| ŅõĄė×Ó·ūŗÅ | ĪļÖŹµÄĮæÅضČ(mol”¤L£1) |
| | |
| | |
| | |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
A”¢B”¢C”¢D”¢E”¢F”¢GĘßÖÖĪļÖŹ¼ä“ęŌŚČēĶ¼ĖłŹ¾µÄ×Ŗ»Æ¹ŲĻµ£¬ĘäÖŠA”¢B”¢D”¢Gŗ¬ÓŠĶ¬ÖÖŌŖĖŲ”£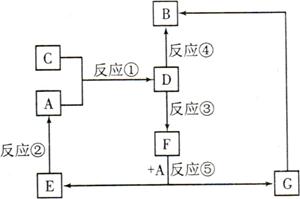
ŅŃÖŖ£ŗ
AĪŖ½šŹōµ„ÖŹ£¬BĪŖŗģŗÖÉ«¹ĢĢ壬EĪŖĆܶČ×īŠ”µÄĘųĢ壬GĪŖĒ³ĀĢÉ«µÄČÜŅŗ”£
DµÄĖ®ČÜŅŗĪŖ»ĘÉ«ČÜŅŗ£¬ÄÜÓėĻõĖįŅųČÜŅŗ·“Ӧɜ³É²»ČÜÓŚĻ”ĻõĖįµÄ°×É«³Įµķ”£
ŌŚĖ®ČÜŅŗÖŠDÄܽ«Ä³Ńõ»ÆĪļŃõ»ÆĪŖF£¬FŹĒŗ¬ÓŠČżÖÖŌŖĖŲµÄ»ÆŗĻĪļ”£
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©¹¹³ÉCĪļÖŹµÄŌŖĖŲŌŚÖÜĘŚ±ķÖŠµÄĪ»ÖĆŹĒ £¬ŌŚ¶ĢÖÜĘŚÖ÷×åŌŖĖŲÖŠ£¬øĆŌŖĖŲÓėĘäĻąĮŚŌŖĖŲµÄŌ×Ó°ė¾¶“ӓ󵽊”µÄĖ³ŠņŹĒ (ÓĆŌŖĖŲ·ūŗűķŹ¾)”£
£Ø2£©DµÄĖ®ČÜŅŗ³Ź ŠŌ£¬ĒėÓĆĄė×Ó·½³ĢŹ½½āŹĶŌŅņ£ŗ ”£
£Ø3£©ÉĻŹö·“Ó¦ÖŠŹōÓŚÖĆ»»·“Ó¦µÄŹĒ (ĢīŠņŗÅ)”£
£Ø4£©·“Ó¦¢Ū(¼“D½«Ä³ÖÖŃõ»ÆĪļŃõ»ÆĪŖF)µÄĄė×Ó·½³ĢŹ½£ŗ ”£
£Ø5£©¶ŌĘųĢåC½ųŠŠČēĻĀŹµŃ锣ŅŃÖŖÕūøö·“Ó¦¹ż³ĢÖŠ£¬ĆæĻūŗÄ0.1mol KI£¬×ŖŅʵĵē×ÓŹżŌ¼ĪŖ3.612”Į1023øö”£Ēė°“ÕÕŅŖĒóĢīæÕ£ŗ
| ŹµŃé²½Öč | ŹµŃéĻÖĻó | Š“Ąė×Ó·½³ĢŹ½ |
| ½«ÉŁĮæĘųĢåĶØČėµķ·ŪKIČÜŅŗ | ČÜŅŗ×ī³õ±ä³É É« | |
| ¼ĢŠųĶØČėĘųĢå | ČÜŅŗÖš½„±ä³ÉĪŽÉ« | |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ij¹¤Ņµ·ĻĖ®½öŗ¬ĻĀ±ķÖŠµÄijŠ©Ąė×Ó£¬ĒŅø÷ÖÖĄė×ÓµÄĪļÖŹµÄĮæÅضČĻąµČ£¬¾łĪŖ0.1 mol/L(“ĖŹżÖµŗöĀŌĖ®µÄµēĄė¼°Ąė×ÓµÄĖ®½ā)”£
| ŃōĄė×Ó | K£«””Ag£«””Mg2£«””Cu2£«””Al3£«””NH4+ |
| ŅõĄė×Ó | Cl£””CO32”Ŗ””NO3”Ŗ””SO42”Ŗ””I£ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
¢ń.ĶĢś¼°Ęä»ÆŗĻĪļŌŚČÕ³£Éś»īÖŠÓ¦ÓĆ¹ć·ŗ£¬Ä³ŃŠ¾æŠŌѧĻ°Š”×éÓĆ“ÖĶ£Øŗ¬ŌÓÖŹFe£©Óė¹żĮæĀČĘų·“Ó¦µĆ¹ĢĢåA£¬ÓĆĻ”ŃĪĖįČܽāA£¬Č»ŗó¼ÓŹŌ¼Įµ÷½ŚČÜŅŗµÄpHŗóµĆČÜŅŗB£¬ČÜŅŗB¾ĻµĮŠ²Ł×÷æɵĆĀČ»ÆĶ¾§Ģ壬Ēė»Ų“š£ŗ
£Ø1£©¹ĢĢåAÓĆĻ”ŃĪĖįČܽāµÄŌŅņŹĒ __”£
£Ø2£©¼ģŃéČÜŅŗBÖŠŹĒ·ń“ęŌŚFe3£«µÄ·½·ØŹĒ __”£
£Ø3£©ŅŃÖŖŌŖĖŲŌŚøß¼ŪĢ¬Ź±³£±ķĻÖŃõ»ÆŠŌ£¬ČōŌŚĖįŠŌCuSO4ČÜŅŗÖŠ¼ÓČėŅ»¶ØĮæµÄNa2SO3ŗĶNaClČÜŅŗ£¬¼ÓČČ£¬Éś³ÉCuCl³Įµķ£¬ŌņÉś³ÉCuClµÄĄė×Ó·½³ĢŹ½ŹĒ ”£
¢ņ.³£ĪĀĻĀ£¬Ä³Ķ¬Ń§½«Ļ”ŃĪĖįŗĶ°±Ė®µČĢå»ż»ģŗĻ£¬Į½ÖÖČÜŅŗµÄÅضČŗĶ»ģŗĻŗóĖłµĆČÜŅŗµÄpHČēĻĀ±ķ£ŗ
| ŹµŃ鱹ŗÅ | °±Ė®ĪļÖŹµÄĮæÅضČ/£Ømol”¤L£1£© | ŃĪĖįĪļÖŹµÄĮæÅضČ/£Ømol”¤L£1£© | »ģŗĻČÜŅŗpH |
| ¢Ł | 0.1 | 0.1 | pH£½5 |
| ¢Ś | c | 0.2 | pH£½7 |
| ¢Ū | 0.2 | 0.1 | pH>7 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
1 Lij»ģŗĻČÜŅŗ£¬æÉÄÜŗ¬ÓŠµÄĄė×ÓČēĻĀ±ķ”£
| æÉÄÜ“óĮæŗ¬ÓŠµÄŃōĄė×Ó | H£«”¢K£«”¢Mg2£«”¢Al3£«”¢NH4+”¢Fe2£«”¢Fe3£« |
| æÉÄÜ“óĮæŗ¬ÓŠµÄŅõĄė×Ó | Cl£”¢Br£”¢I£”¢CO32”Ŗ”¢AlO2”Ŗ |
| Cl2µÄĢå»ż(±ź×¼×“æö) | 2.8 L | 5.6 L | 11.2 L |
| n(Cl£) | 1.25 mol | 1.5 mol | 2 mol |
| n(Br£) | 1.5 mol | 1.4 mol | 0.9 mol |
| n(I£) | a mol | 0 | 0 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ĀČĘų¼°Ęä»ÆŗĻĪļŌŚ¹¤Å©ŅµÉś²śŗĶČĖĄąÉś»īÖŠ¶¼ÓŠ×ÅÖŲŅŖµÄÓ¦ÓĆ”£
(1)Ca(ClO)2”¢NaClO”¢NaClO2µČŗ¬ĀČ»ÆŗĻĪļ¶¼ŹĒ³£ÓƵÄĻū¶¾¼ĮŗĶĘÆ°×¼Į,ŹĒŅņĪŖĖüĆĒ¶¼¾ßÓŠ””””””””””ŠŌ,ĒėŠ“³ö¹¤ŅµÉĻÓĆĀČĘųÓėNaOHČÜŅŗ·“Ӧɜ²śĻū¶¾¼ĮNaClOµÄĄė×Ó·½³ĢŹ½”””””””””””””””””””””””””””””””£
(2)×Ų»ĘÉ«Ēæ“Ģ¼¤ŠŌĘųĢåCl2OĪŖ¹ś¼Ź¹«ČĻøߊ§°²Č«Ćš¾śĻū¶¾¼ĮÖ®Ņ»,ŹµŃéŹŅæÉÓĆ³±ŹŖµÄCl2ÓėNa2CO3·“Ó¦ÖĘȔɣĮæCl2OŗĶNaHCO3µÄĄė×Ó·½³ĢŹ½”””””””””””””””””””””””””””””””””””£
(3)»ĘÉ«ĘųĢåClO2æÉÓĆÓŚĪŪĖ®É±¾śŗĶŅūÓĆĖ®¾»»Æ”£
¢ŁKClO3ÓėSO2ŌŚĒæĖįŠŌČÜŅŗÖŠ·“Ó¦æÉÖʵĆClO2,“Ė·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ”””””””””””””””””””””””””””£
¢ŚClO2ÓöÅØŃĪĖį»įÉś³ÉCl2,ĆæÉś³É1 mol Cl2×ŖŅʵē×ÓµÄĪļÖŹµÄĮæĪŖ”””””””””£
¢ŪClO2æɽ«·ĻĖ®ÖŠµÄMn2+×Ŗ»ÆĪŖMnO2¶ų³żČ„,±¾Éķ»¹ŌĪŖCl-,øĆ·“Ó¦¹ż³ĢÖŠŃõ»Æ¼ĮÓė»¹Ō¼ĮµÄĪļÖŹµÄĮæÖ®±ČĪŖ”””””””””£
(4)ÓĆCl2Éś²śÄ³Š©ŗ¬ĀČÓŠ»śĪļŹ±»į²śÉśø±²śĪļHCl”£ĄūÓĆ·“Ó¦4HCl+O2 2Cl2+2H2O,æÉŹµĻÖĀȵÄŃ»·ĄūÓĆ”£
2Cl2+2H2O,æÉŹµĻÖĀȵÄŃ»·ĄūÓĆ”£
ŅŃÖŖ:¢ŁÉĻŹö·“Ó¦ÖŠ,4 mol HCl±»Ńõ»Æ·Å³ö115.6 kJµÄČČĮ攣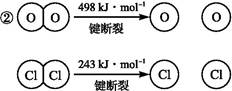
Ōņ¶ĻæŖ1 mol H”ŖO¼üÓė¶ĻæŖ1 mol H”ŖCl¼üĖłŠčÄÜĮæĻą²īŌ¼ĪŖ”””””””” kJ,H2OÖŠH”ŖO¼ü±ČHClÖŠH”ŖCl¼ü(Ģī”°Ēæ”±»ņ”°Čõ”±)”””””””””£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com