
”¾ĢāÄæ”æ°“ŅŖĒóĢīæÕ:
(1)ijĶ¬Ń§ŅĄ¾ŻŃõ»Æ»¹Ō·“Ó¦£ŗ2Ag++Cu=Cu2++2AgÉč¼ĘµÄŌµē³ŲČēĶ¼ĖłŹ¾£ŗ
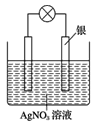
¢Łøŗ¼«·¢ÉśµÄµē¼«·“Ó¦ĪŖ___________£»
¢Śµē½āÖŹČÜŅŗÖŠµÄNO3-Ļņ__________µē¼«ŅĘ¶Æ£»(Š“³öµē¼«²ÄĮĻµÄĆū³Ę)
(2)µ±·“Ó¦½ųŠŠµ½Ņ»¶ĪŹ±¼äŗóČ”³öµē¼«²ÄĮĻ£¬²āµĆijŅ»µē¼«ŌöÖŲĮĖ5.4g£¬ŌņøĆŌµē³Ų·“Ó¦¹²×ŖŅʵĵē×ÓŹżÄæŹĒ________£»
(3)Ė®ŹĒÉśĆüÖ®Ō“£¬Ņ²ŹĒ»Æѧ·“Ó¦ÖŠµÄÖ÷½Ē”£Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
ŅŃÖŖ£ŗ2mol H2ĶźČ«Č¼ÉÕÉś³ÉŅŗĢ¬Ė®Ź±·Å³ö572 kJµÄČČĮ攣
¢ŁČō2molĒāĘųĶźČ«Č¼ÉÕÉś³ÉĖ®ÕōĘų£¬Ōņ·Å³öµÄČČĮæ______(Ģī”°>”±”°<”±»ņ”°=”±)572 kJ£»
¢ŚĆææĖĒāĘųČ¼ÉÕÉś³ÉŅŗĢ¬Ė®Ź±·Å³öµÄČČĮæĪŖ________£»
(4)ĢģČ»Ęų(Ö÷ŅŖ³É·ÖCH4)ŗĶŃõĘų·“Ӧɜ³É¶žŃõ»ÆĢ¼ŗĶĖ®£¬øĆ·“Ó¦ĪŖ·ÅČȵÄŃõ»Æ»¹Ō·“Ó¦£¬æɽ«ĘäÉč¼Ę³ÉČ¼ĮĻµē³Ų£¬¹¹ŌģČēĶ¼ĖłŹ¾£¬a”¢bĮ½øöµē¼«¾łÓɶąæ×µÄĢ¼æé×é³É”£
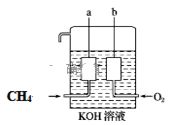
aµē¼«µÄµē¼«·“Ó¦Ź½ŹĒ_________”£
”¾“š°ø”æCu2e=Cu2+ Ķ 0.05NA(»ņ3.01”Į1022) < 143kJ CH48e+10OH=CO32-+7H2O
”¾½āĪö”æ
(1)¢Łøł¾Żµē³Ų·“Ó¦Ź½ÖŖ£¬Ź§µē×Ó»ÆŗĻ¼ŪÉżøߵĽšŹō×÷øŗ¼«£¬øŗ¼«·¢ÉśŃõ»Æ·“Ó¦£»
¢ŚČÜŅŗÖŠµÄŅõĄė×ÓĻņÕżµēŗɽĻ¶ąµÄøŗ¼«ŅĘ¶Æ£¬
(2)øł¾Żµē¼«·“Ó¦Ź½¼ĘĖćĶعżµē×ÓµÄĪļÖŹµÄĮ棻
(3)¢ŁĻąĶ¬ÖŹĮæµÄĶ¬Ņ»ĪļÖŹ£¬ĘųĢåŗ¬ÓŠµÄÄÜĮæ±ČŅŗĢ¬¶ą£¬ŅŗĢ¬±Č¹ĢĢ¬¶ą·ÖĪöÅŠ¶Ļ£»
¢Ś¼ĘĖć2molĒāĘųµÄÖŹĮæ£¬Č»ŗóøł¾Ż·“Ó¦ĪļµÄÖŹĮæÓė·“Ó¦·Å³öµÄČČĮæ³ŹÕż±Č¼ĘĖć·Å³öČČĮæ¶ąÉŁ£»
(4)ĶØČėČ¼ĮĻµÄµē¼«ĪŖøŗ¼«£¬Ź§Č„µē×Ó£¬·¢ÉśŃõ»Æ·“Ó¦£¬·“Ó¦²śĪļŅŖ½įŗĻµē½āÖŹČÜŅŗµÄĖį¼īŠŌ·ÖĪö”£
(1)¢Łøł¾Ż·“Ó¦2Ag++Cu=Cu2++2AgæÉÖŖ£ŗCuŹ§Č„µē×Ó£¬·“Ó¦Ńõ»Æ·“Ó¦£¬Ņņ“Ė×÷Ōµē³ŲµÄøŗ¼«£¬µē¼«·“Ó¦Ź½ĪŖCu2e=Cu2+£»
¢Śøł¾ŻĶ¬ÖÖµēŗÉĻą»„Åųā£¬ŅģÖÖµēŗÉĻą»„ĪüŅżµÄŌŌņ£¬ČÜŅŗÖŠµÄNO3-»įĻņÕżµēŗɽĻ¶ąµÄøŗ¼«Cuµē¼«·½ĻņŅĘ¶Æ£»ŌŚŅõ¼«·¢Éś·“Ó¦£ŗAg++e-=Ag£¬n(Ag)=n(e-)=5.4g”Ā108g/mol=0.05mol£¬ĖłŅŌµē×Ó×ŖŅĘŹżÄæĪŖ0.05NA£»
(3)¢Ł2mol H2ĶźČ«Č¼ÉÕÉś³ÉŅŗĢ¬Ė®Ź±·Å³ö572 kJµÄČČĮ棬Čō2mol H2ĶźČ«Č¼ÉÕÉś³ÉĘųĢ¬Ė®£¬ÓÉÓŚµČÖŹĮæµÄĖ®ÕōĘųŗ¬ÓŠµÄÄÜĮæ±ČŅŗĢ¬Ė®øߣ¬Ņņ“Ė·“Ó¦·Å³öµÄČČĮæ±Č572 kJµÄČČĮæÉŁ£»
¢Ś2mol H2ĶźČ«Č¼ÉÕÉś³ÉŅŗĢ¬Ė®Ź±·Å³ö572 kJµÄČČĮ棬2molH2µÄÖŹĮæĪŖ4g£¬Ōņ1g H2ĶźČ«Č¼ÉÕÉś³ÉŅŗĢ¬Ė®Ź±·Å³öµÄČČĮæĪŖ572 kJ”Ā4=143kJ£»
(4)øł¾ŻĶ¼Ź¾æÉÖŖaµē¼«ŹĒĶØČėČ¼ĮĻµÄµē¼«£¬ĪŖøŗ¼«£¬¼×Ķ鏧Ȅµē×Ó£¬ÓÉÓŚµē½āÖŹČÜŅŗĪŖKOHČÜŅŗ£¬ĻŌ¼īŠŌ£¬ĖłŅŌaµē¼«µÄµē¼«·“Ó¦Ź½ŹĒCH48e-+10OH=CO32-+7H2O”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æČōĘųĢ¬ĢžDµÄĆܶČĪŖ1.16 g”¤L£1(±ź×¼×“æö)£¬øł¾ŻĻĀĮŠ×Ŗ»Æ¹ŲĻµĶʶĻ£ŗA![]() B
B![]() C£»A
C£»A![]() D
D![]() Eӣ
Eӣ
(1)Š“³öA”«EµÄ½į¹¹¼ņŹ½£ŗ
A£®________________£¬B.________________£¬C£®________________£¬D.________________£¬
E£®________________”£
(2)Š“³öĻĀĮŠ·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ
AB£ŗ________________________________________________________________________£»
BC£ŗ________________________________________________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”涔ĶéC4H10µÄ¶žĀČ“śĪļ¹²(²»æ¼ĀĒĮ¢ĢåŅģ¹¹)
A.9ÖÖB.6 ÖÖC.5ÖÖD.8ÖÖ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æČēĶ¼ĖłŹ¾£¬aÖŠ·ÅÖĆø½×ÅŃõ»ÆĶ·ŪÄ©µÄŹÆĆŽČŽ£¬ĻņaÖŠ³ÖŠųĶØČėĘųĢ¬ĪļÖŹX£¬æÉŅŌ¹Ū²ģµ½ŹÆĆŽČŽÉĻŗŚÉ«·ŪÄ©±ä³ÉŗģÉ«¹ĢĢ¬ĪļÖŹ£¬Ķ¬Ź±c“¦µÄUŠĪ¹ÜÖŠÓŠĪŽÉ«ŅŗĢåÉś³É(¼ŁÉčXĘųĢåČ«²æ·“Ó¦£¬ø÷“¦·“Ó¦¾łĶźČ«)”£
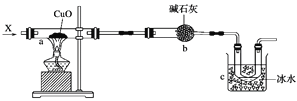
(1)Š“³öa“¦·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ_______________________________________________”£
(2)c“¦ŅŗĢåÖ÷ŅŖ³É·ÖµÄ½į¹¹¼ņŹ½ĪŖ__________________________£»¼ģŃéøĆĪļÖŹµÄ²Ł×÷²½ÖčŹĒ_________________________________£¬ĘäĻÖĻóĪŖ______________________________£»ÓŠ¹Ų·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ____________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æ¹āæĢ½ŗµÄŅ»ÖÖŗĻ³ÉĀ·ĻßČēĻĀ£ŗ

ŅŃÖŖ£ŗ
I.![]()
II.![]()
III.RCOOH+CH![]() CH
CH![]() RCOOCH=CH2
RCOOCH=CH2
»Ų“šĻĀĮŠĪŹĢā
(1)AµÄĆū³ĘŹĒ______”£CÖŠĖłŗ¬Ńõ¹ŁÄÜĶŵÄĆū³ĘŹĒ_______”£C”śDµÄ·“Ó¦ĄąŠĶŹĒ________”£
(2)BŗĶŅų°±ČÜŅŗ·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ_______________”£
(3)D+G”ś¹āæĢ½ŗµÄ»Æѧ·½³ĢŹ½ĪŖ_________________”£
(4)TŹĒCµÄĶ¬·ÖŅģ¹¹Ģ壬T¾ßÓŠĻĀĮŠŠŌÖŹ»ņĢŲÕ÷£ŗ¢ŁÄÜ·¢ÉśĖ®½ā·“Ó¦ŗĶŅų¾µ·“Ó¦£»¢ŚÄÜŹ¹äåµÄĖÄĀČ»ÆĢ¼ĶŹÉ«£»¢ŪŹōÓŚ·¼Ļć×å»ÆŗĻĪļ”£ŌņTµÄ½į¹¹ÓŠ____ÖÖ”£ĘäÖŠŗĖ“Ź²ÕńĒāĘ×ĪŖ5×é·å£¬ĒŅ·åĆ껿±ČĪŖ1”Ć1”Ć2”Ć2”Ć2µÄ½į¹¹¼ņŹ½ĪŖ_______”£
(5)øł¾ŻŅŃÓŠÖŖŹ¶²¢½įŗĻĻą¹ŲŠÅĻ¢£¬Š“³öŅŌCH3CH2OHĪŖŌĮĻÖʱøCH3CH2CH2COOHµÄŗĻ³ÉĀ·ĻßĮ÷³ĢĶ¼_______(ĪŽ»śŹŌ¼ĮČĪÓĆ)”£
£ØŗĻ³ÉĀ·ĻßĮ÷³ĢĶ¼Ź¾ĄżČēĻĀ£ŗCH2=CH2![]() CH3CH2Br
CH3CH2Br![]() CH3CH2OH£©
CH3CH2OH£©
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĻĀĮŠĖµ·Ø²»ÕżČ·µÄŹĒ£Ø £©
A. ”°³ōŃõæÕ¶“”±”¢”°¹ā»ÆѧŃĢĪķ”±”¢”°ĻõĖįŠĶĖįÓź”±µÄŠĪ³É¶¼ÓėµŖŃõ»ÆŗĻĪļÓŠ¹Ų
B. ¹¤Ņµŗ£Ė®ÖĘČ”Ć¾Į÷³Ģ£ŗŗ£Ė®![]() Mg(OH)2”śMgCl2
Mg(OH)2”śMgCl2![]() Mg
Mg
C. ĶĘ¹ćŹ¹ÓĆŅŅ“¼ĘūÓĶ“śĢęĘūÓĶÄæµÄŹĒĪŖĮĖ¼õÉŁĪĀŹŅĘųĢåµÄÅÅ·Å
D. ¹¤ŅµÉś²ś²£Į§”¢Ė®Äą¶¼ÓĆŹÆ»ŅŹÆ×öŌĮĻ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĻĀĮŠŠšŹöÓėĶ¼Ļó¶ŌÓ¦·ūŗĻµÄŹĒ(””””)

A. ¶ŌÓŚ“ļµ½Ę½ŗāדĢ¬µÄN2(g)£«3H2(g)![]() 2NH3(g)ŌŚt0Ź±æĢ³äČėĮĖŅ»¶ØµÄNH3£¬Ę½ŗāÄęĻņŅʶÆ
2NH3(g)ŌŚt0Ź±æĢ³äČėĮĖŅ»¶ØµÄNH3£¬Ę½ŗāÄęĻņŅʶÆ
B. P2>P1£¬T1>T2
C. øĆĶ¼Ļó±ķŹ¾µÄ·½³ĢŹ½ĪŖ£ŗ2A===B£«3C
D. ¶ŌÓŚ·“Ó¦2X(g)£«3Y(g)![]() 2Z(g)””¦¤H<0£¬yæÉŅŌ±ķŹ¾YµÄ°Ł·Öŗ¬Įæ
2Z(g)””¦¤H<0£¬yæÉŅŌ±ķŹ¾YµÄ°Ł·Öŗ¬Įæ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĻĀĮŠÓŠ¹ŲŹµŃéµÄŃ”ĻīÕżČ·µÄŹĒ£Ø £©
A.  ÅäÖĘ0.10mol/LNaOHČÜŅŗ
ÅäÖĘ0.10mol/LNaOHČÜŅŗ
B. 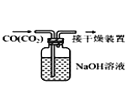 ³żČ„COÖŠµÄCO2
³żČ„COÖŠµÄCO2
C. 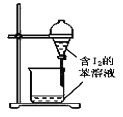 ±½ŻĶČ”µāĖ®ÖŠµÄI2£¬·Ö³öĖ®²ćŗóµÄ²Ł×÷
±½ŻĶČ”µāĖ®ÖŠµÄI2£¬·Ö³öĖ®²ćŗóµÄ²Ł×÷
D. 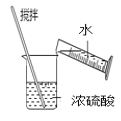 Ļ”ŹĶÅØĮņĖį
Ļ”ŹĶÅØĮņĖį
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æÖŹ×ÓŗĖ“Ź²ÕńĘ×£ØPMR£©ŹĒŃŠ¾æÓŠ»śĪļ½į¹¹µÄÓŠĮ¦ŹÖ¶ĪÖ®Ņ»”£ŌŚĖłŃŠ¾æµÄ»ÆŗĻĪļ·Ö×ÓÖŠ£¬ĆæŅ»½į¹¹ÖŠµÄµČŠŌĒāŌ×ÓŌŚPMRĘ×ÖŠ¶¼øų³öĮĖĻąÓ¦µÄ·å£ØŠÅŗÅ£©”£Ę×ÖŠ·åµÄĒæ¶ČÓė½į¹¹ÖŠµÄHŌ×ÓŹż³ÉÕż±Č”£ĄżČē£ŗŅŅČ©µÄ½į¹¹Ź½ĪŖ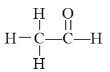 £¬ĘäÖŠPMRĘ×ÖŠÓŠ2øöŠÅŗŷ壬ĘäĒæ¶ČÖ®±ČĪŖ3”Ć1”£
£¬ĘäÖŠPMRĘ×ÖŠÓŠ2øöŠÅŗŷ壬ĘäĒæ¶ČÖ®±ČĪŖ3”Ć1”£
£Ø1£©·Ö×ÓŹ½ĪŖC3H6O2µÄ¶žŌŖ»ģŗĻĪļ£¬ČōŌŚPMRĘ×ÉĻ¹Ū²ģµ½ĒāŌ×Óøų³öµÄ·åÓŠĮ½ÖÖĒéæö”£µŚŅ»ÖÖĒéæö·åµÄĒæ¶ČÖ®±ČĪŖ3”Ć3£»µŚ¶žÖÖĒéæö·åµÄĒæ¶ČÖ®±ČĪŖ3”Ć2”Ć1”£ÓÉ“ĖĶʶĻæÉÄÜ×é³ÉøĆ»ģŗĻĪļµÄø÷ĪļÖŹŹĒ£ØŠ“½į¹¹¼ņŹ½£©£ŗ__________________”¢__________”¢_______________£»
£Ø2£©ŌŚ²āµĆµÄPMRĘ×ÉĻæɹŪ²ģµ½»ÆŗĻĪļ![]() ÓŠ3øö·å£¬¶ųCH3CH£½CHCl»ÆŗĻĪļČ“µĆµ½ĒāŌ×Óøų³öµÄ6øöŠÅŗŷ唣øł¾ŻŌ×ÓŌŚæÕ¼äµÄÅÅĮŠ·½Ź½µÄ²»Ķ¬£¬Š“³öCH3CH£½CHCl·Ö×ÓµÄæÕ¼äŅģ¹¹Ģå£ŗ_____________________________________”£
ÓŠ3øö·å£¬¶ųCH3CH£½CHCl»ÆŗĻĪļČ“µĆµ½ĒāŌ×Óøų³öµÄ6øöŠÅŗŷ唣øł¾ŻŌ×ÓŌŚæÕ¼äµÄÅÅĮŠ·½Ź½µÄ²»Ķ¬£¬Š“³öCH3CH£½CHCl·Ö×ÓµÄæÕ¼äŅģ¹¹Ģå£ŗ_____________________________________”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com