
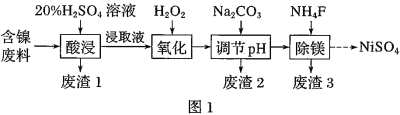
����Ŀ��ij����![]() ��������FeO��
��������FeO��![]() ��MgO��
��MgO��![]() �����ʣ��ô˷�����ȡ
�����ʣ��ô˷�����ȡ![]() �Ĺ���������ͼ1��
�Ĺ���������ͼ1��
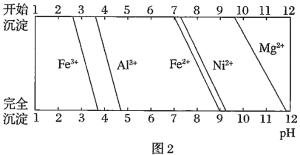
��֪�����йؽ���������������������������pH��ͼ��
��![]() ʱ��
ʱ��![]() �ĵ��볣��
�ĵ��볣��![]() �ĵ��볣��
�ĵ��볣��![]() ��
��![]()
![]() ��
��
(1)��![]() ������Һ��pH��5���õ�����2����Ҫ�ɷ���______
������Һ��pH��5���õ�����2����Ҫ�ɷ���______![]() �ѧʽ
�ѧʽ![]() ��
��
(2)![]() ���뱥��
���뱥��![]() ��Һ��Ӧ����
��Һ��Ӧ����![]() �����û�ѧƽ���ƶ�ԭ������
�����û�ѧƽ���ƶ�ԭ������![]() �ñ�Ҫ�����ֺ����ӷ���ʽ�ش�
�ñ�Ҫ�����ֺ����ӷ���ʽ�ش�![]() ______��
______��
(3)![]() ʱ��
ʱ��![]() ��NaF��Һ��
��NaF��Һ��![]() ______
______![]() �г�����ʽ����
�г�����ʽ����![]() ��Һ��______
��Һ��______![]() ������������������������������
������������������������������![]() ��
��
(4)��֪����ǰ��Һ��![]() ������þ�ʴﵽ
������þ�ʴﵽ![]() ʱ����Һ��
ʱ����Һ��![]() ______
______![]() ��
��
(5)��NaOH��Һ����NaClO��![]() ��Ӧ�ɵ�
��Ӧ�ɵ�![]() ����ѧ����ʽΪ____________��
����ѧ����ʽΪ____________��![]() ������������Ͻ�����������Ե��
������������Ͻ�����������Ե��![]() ��Һ
��Һ![]() ������ԭ��Ϊ��
������ԭ��Ϊ��![]() �������ĵ缫��Ӧʽ��______��
�������ĵ缫��Ӧʽ��______��
���𰸡�![]() ��
��![]() �Ȼ��ˮ�����������һˮ�ϰ���
�Ȼ��ˮ�����������һˮ�ϰ���![]() ��þ�������ӷ�Ӧ����������������Ũ�ȼ�С���ٽ�ƽ��������У����ɵ�һˮ�ϰ��ֽ����ɰ�����
��þ�������ӷ�Ӧ����������������Ũ�ȼ�С���ٽ�ƽ��������У����ɵ�һˮ�ϰ��ֽ����ɰ�����![]()
![]() ����
���� ![]()
![]()
![]()
��������
ijNiO�ķ�������FeO��![]() ��MgO��
��MgO��![]() �����ʣ�����ϡ�����ܽ����˵õ�����1Ϊ
�����ʣ�����ϡ�����ܽ����˵õ�����1Ϊ![]() ����ҺΪ
����ҺΪ![]() ��
��![]() ��
��![]() ��
��![]() �������������������������Ϊ�����ӣ��ټ���̼������Һ������ҺpH��ʹ�����ӣ�������ȫ�����������˵õ�����2Ϊ��������������������������Һ�м���
�������������������������Ϊ�����ӣ��ټ���̼������Һ������ҺpH��ʹ�����ӣ�������ȫ�����������˵õ�����2Ϊ��������������������������Һ�м���![]() ����
����![]() �����ɳ�������3Ϊ
�����ɳ�������3Ϊ![]() �����˵õ�����Һ����Һ�л��
�����˵õ�����Һ����Һ�л��![]() ����ķ�����ͨ������Ũ������ȴ�ᾧ������ϴ�ӣ�����õ����壬ʧȥ�ᾧˮ�õ����������ݴ˷�����
����ķ�����ͨ������Ũ������ȴ�ᾧ������ϴ�ӣ�����õ����壬ʧȥ�ᾧˮ�õ����������ݴ˷�����
ijNiO�ķ�������FeO��![]() ��MgO��
��MgO��![]() �����ʣ�����ϡ�����ܽ����˵õ�����1Ϊ
�����ʣ�����ϡ�����ܽ����˵õ�����1Ϊ![]() ����ҺΪ
����ҺΪ![]() ��
��![]() ��
��![]() ��
��![]() �������������������������Ϊ�����ӣ��ټ���̼������Һ������ҺpH��ʹ�����ӣ�������ȫ�����������˵õ�����2Ϊ��������������������������Һ�м���
�������������������������Ϊ�����ӣ��ټ���̼������Һ������ҺpH��ʹ�����ӣ�������ȫ�����������˵õ�����2Ϊ��������������������������Һ�м���![]() ����
����![]() �����ɳ�������3Ϊ
�����ɳ�������3Ϊ![]() �����˵õ�����Һ����Һ�л��
�����˵õ�����Һ����Һ�л��![]() ����ķ�����ͨ������Ũ������ȴ�ᾧ������ϴ�ӣ�����õ����壬ʧȥ�ᾧˮ�õ���������
����ķ�����ͨ������Ũ������ȴ�ᾧ������ϴ�ӣ�����õ����壬ʧȥ�ᾧˮ�õ���������
![]() ����̼������Һ������ҺpH��ʹ�����ӣ�������ȫ�����������������������������������õ�����2����Ҫ�ɷ���
����̼������Һ������ҺpH��ʹ�����ӣ�������ȫ�����������������������������������õ�����2����Ҫ�ɷ���![]() ��
��![]() ��
��
�ʴ�Ϊ��![]() ��
��![]() ��
��
![]() ���뱥��
���뱥��![]() ��Һ��Ӧ����
��Һ��Ӧ����![]() ���Ȼ��ˮ�����������һˮ�ϰ���
���Ȼ��ˮ�����������һˮ�ϰ���![]() ��þ�������ӷ�Ӧ����������������Ũ�ȼ�С���ٽ�ƽ��������У����ɵ�һˮ�ϰ��ֽ����ɰ�����
��þ�������ӷ�Ӧ����������������Ũ�ȼ�С���ٽ�ƽ��������У����ɵ�һˮ�ϰ��ֽ����ɰ�����![]() ��
��
�ʴ�Ϊ���Ȼ��ˮ�����������һˮ�ϰ���![]() ��þ�������ӷ�Ӧ����������������Ũ�ȼ�С���ٽ�ƽ��������У����ɵ�һˮ�ϰ��ֽ����ɰ�����
��þ�������ӷ�Ӧ����������������Ũ�ȼ�С���ٽ�ƽ��������У����ɵ�һˮ�ϰ��ֽ����ɰ�����![]() ��
��
![]() ʱ��
ʱ��![]() ��NaF��Һ��
��NaF��Һ��![]() ����ϵ���ƽ�ⳣ��
����ϵ���ƽ�ⳣ��![]() ��
�� ��ˮ��ƽ��ʱ
��ˮ��ƽ��ʱ![]() ����ȡ
����ȡ![]() ��
��![]() ����
����![]() ��һˮ�ϰ�����ƽ�ⳣ��
��һˮ�ϰ�����ƽ�ⳣ��![]() ��HF�ĵ��볣��
��HF�ĵ��볣��![]() ��
��![]() ����
����![]() ��Һ��笠�����ˮ��̶ȴ���Һ�����ԣ�
��Һ��笠�����ˮ��̶ȴ���Һ�����ԣ�
�ʴ�Ϊ��![]() �����ԣ�
�����ԣ�
![]() ��֪����ǰ��Һ��
��֪����ǰ��Һ��![]() ������þ�ʴﵽ
������þ�ʴﵽ![]() ʱ��
ʱ��![]() ��
��![]() ��
��![]() ��
��![]() ��
��
�ʴ�Ϊ��![]() ��
��
![]() ��NaOH��Һ����NaClO��
��NaOH��Һ����NaClO��![]() ��Ӧ�ɵ�
��Ӧ�ɵ�![]() ��ͬʱ���������ƺ��Ȼ��ƣ���Ӧ�Ļ�ѧ����ʽΪ��
��ͬʱ���������ƺ��Ȼ��ƣ���Ӧ�Ļ�ѧ����ʽΪ��![]() ��
��![]() ������������Ͻ�����������Ե��
������������Ͻ�����������Ե��![]() ��Һ
��Һ![]() ������ԭ��Ϊ��
������ԭ��Ϊ��![]()
![]()
![]() ��������
��������![]() ʧ��������
ʧ��������![]() ���缫��ӦΪ��
���缫��ӦΪ��![]() ��
��
�ʴ�Ϊ��![]() ��
��![]() ��
��


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������һ�ֿ��Է�����硢�ŵ��װ�á���һ�������ڳ��ͷŵ�ʱ�����ķ�ӦΪNiO2+ Fe+2H2O![]() Fe(OH)2+Ni(OH)2�������йظõ�ص�˵������ȷ����
Fe(OH)2+Ni(OH)2�������йظõ�ص�˵������ȷ����
A.�ŵ�ʱ�������Һ��ǿ����
B.���ʱ���巴ӦΪNi(OH)2+2OH--2e-=NiO2+2H2O
C.�ŵ�ʱ����������ҺpH��С
D.���ʱ����������Һ�ļ��Ա��ֲ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʹ��ʯ�����ѽ�ĸ������еļ�������ȡ��������Ҫ���������У��䷴Ӧ�����е������仯��ͼ��ʾ��
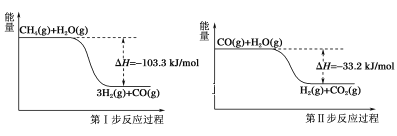
������ˮ������Ӧ���ɶ�����̼���������Ȼ�ѧ����ʽΪ�� ��
A.CH4(g)��H2O(g)=3H2(g)��CO(g) ��H����103.3kJ/mol
B.CH4(g)��2H2O(g)=4H2(g)��CO2(g) ��H����70.1kJ/mol
C.CH4(g)��2H2O(g)=4H2(g)��CO2(g) ��H��70.1kJ/mol
D.CH4(g)��2H2O(g)=4H2(g)��CO2(g) ��H����136.5kJ/mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����7�֣����������ʣ�
��ʯī�����������ƾ�������ˮ����������̼����̼�����ƹ��壻
������������Һ����������������ƹ��壻���Ȼ������塣
��1�������ܵ�����������������������ڷǵ���ʵ�������������������
����ǿ����ʵ���������������������������ʵ�������������������
��2��д������������ˮ�ĵ��뷽��ʽ����������������������������������
��3��д��������������ˮ�з�Ӧ�����ӷ���ʽ������������������������������
��4�������������Ƴ���Һ����μ�������Һ�������������д�����ӷ���ʽ��������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���屽��һ�ֻ���ԭ�ϣ�ʵ���Һϳ��屽��װ��ʾ��ͼ���й��������£�


�����ºϳɲ���ش����⣺
(1)��a�м���15 mL��ˮ����������м����b��С�ļ���4.0 mLҺ̬�壬��a�е��뼸���壬�а�������������Ϊ������_____���壬�����μ���Һ����꣬װ��d��������______��
(2)Һ�����������в�������ᴿ��
����a�м���10 mLˮ��Ȼ����˳�ȥδ��Ӧ����м��
����Һ������10 mLˮ��8 mL10����NaOH��Һ��10 mLˮϴ�ӣ�NaOH��Һϴ�ӵ�������_____��
����ֳ��Ĵ��屽�м�����������ˮ�Ȼ��ƣ����á����ˣ������Ȼ��Ƶ�Ŀ����_____��
(3)������������������屽�л����е���Ҫ����Ϊ_______��Ҫ��һ���ᴿ�����в����б������_______(������ȷѡ��ǰ����ĸ)��
A. �ؽᾧ B. ���� C. ���� D. ��ȡ
(4)�ڸ�ʵ���У�a���ݻ����ʺϵ���_____(������ȷѡ��ǰ����ĸ)��
A. 25 mL B.50 mL C. 250 mL D. 500 mL
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪��![]() ʱ
ʱ
��ѧʽ |
|
|
|
����ƽ�ⳣ�� |
|
|
|
����˵����ȷ���� ( )
A. ����ϡ�����У�![]() ��С
��С
B. ![]() ��Һ�У�
��Һ�У�![]()
C. ������HCN��Һ�м���![]() ,������
,������![]()
D. ���ʵ���Ũ����ͬʱ![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijѧ����0.100 mol��L-1��NaOH����Һ�ζ�δ֪Ũ�ȵ����ᣬ������ɷֽ�Ϊ���¼�����
A����ȡ20.00 mL����������Һע��ྻ����ƿ��������2��3�η�̪��
B���ñ���Һ��ϴ�ζ���2��3�Σ�
C����ʢ�б���Һ�ļ�ʽ�ζ��̶ܹ��ã����ڵζ��ܼ���ʹ֮������Һ��
D��ȡ��NaOH��Һע���ʽ�ζ������̶ȡ�0������2��3 mL��
E������Һ������0����0�����¿̶ȣ����¶�����
F������ƿ���ڵζ��ܵ����棬�ñ�NaOH��Һ�ζ����յ㲢���µζ���Һ��Ŀ̶ȡ�
�ش��������⣺
��1����ȷ���������˳����(����ĸ�����д)_________��
��2����ȥ��ʽ�ζ��������ݵķ���Ӧ������ͼ�����е�________(����)��Ȼ�����ἷѹ������ʹ���첿�ֳ�����Һ��

��3���ζ�������,�۾�Ӧע��______________��
��4���жϵ���ζ��յ��ʵ��������__________________��
��5�����ݼ�¼���£�
�ζ����� | ������������/mL | ��NaOH��Һ��� | |
�ζ�ǰ�Ŀ̶�/mL | �ζ���Ŀ̶�/mL | ||
��һ�� | 20.00 | 0.40 | 20.50 |
�ڶ��� | 20.00 | 4.10 | 24.00 |
������ | 20.00 | 1.00 | 24.00 |
�����������ݣ��ɼ�����������Ũ��ԼΪ_____________(����С�������λ��)��
��6��������ʵ���У����в���(����������ȷ)����ɲⶨ���ƫ�͵���_____(����ĸ)��
A����ʽ�ζ���ʹ��ǰ��ˮϴ��δ�ô���������ϴ
B����ƿˮϴ��δ����
C����ʽ�ζ��ܼ��첿�������ݣ��ζ�����ʧ
D���ζ��յ����ʱ���Ӷ���
E���ζ��յ����ʱ���Ӷ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ѱ���Ϊ��������֮��ĵ�����������ش��������⣺
(1)���ʯ(TiO2)���ѵ���Ҫ����֮һ����̬Tiԭ�Ӽ۲���ӵ��Ų�ͼΪ_________����̬Oԭ�ӵ���ռ������ܼ��ĵ���������ͼΪ __________�Ρ�
(2)��TiO2Ϊԭ�Ͽ��Ƶ�TiCl4��TiCl4���ۡ��е�ֱ�Ϊ205K��409K�������ڽṹ�������Ƶ�CCl4����Ҫԭ���� __________________��
(3)TiCl4������Ũ�����H2[TiCl6]������Һ�м���NH4ClŨ��Һ��������ɫ��(NH4)2[TiCl6]���塣�þ�����������֮����������� ________��
A�����Ӽ� B�����ۼ� C�����Ӽ������� D����� E�����»���
(4)TiCl4����CH3CH2OH��HCHO��CH3OCH3���л�С�����γɼӺ����������С������Cԭ�ӵ�VSEPRģ�Ͳ�ͬ���������ӵ��� _____���÷�����C�Ĺ���ӻ�����Ϊ________ ��
(5)TiO2��BaCO3һ�����ڿ��Ƶ����ᱵ��
��BaCO3�������ӵ����幹��Ϊ ________��
�ھ�X���߷������������ᱵ�ľ����ṹ����ͼ��ʾ��Ti4+��Ba2+����O2����Ӵ����������ᱵ�Ļ�ѧʽΪ _________����֪�����߳�Ϊa pm��O2���İ뾶Ϊb pm����Ti4+��Ba2+�İ뾶�ֱ�Ϊ____________pm��___________pm��
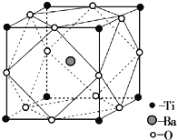
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijѧϰС���Ե�·���ʴҺ�����д���Cu2+��Fe2+��Fe3+��Ϊԭ���Ʊ�����Cu20���Ʊ��������£�

��֪����Cu2O�ڳ�ʪ�Ŀ����л�������������CuO��Ҳ�ױ���ԭΪCu; Cu2O������ˮ���������ڼ�����Һ��Cu2O+2H+ =Cu2++Cu+H2O��
������Cu2O�ķ�Ӧ��4Cu(OH)2+N2H4H2O=2Cu2O+N2��+7H2O
��ش�
��1������II��д������CuR2��Ӧ�����ӷ���ʽ��____________________________
��2������II�����ˮ������ȡ���ϲ���ȡҺ��Ŀ����___________________________
��3������III������ȡ��Ϊ_____________
��4������IV�����Ʊ�����Cu2Oʱ��������Һ��pHΪ5��ԭ����_______________
A.  B.
B.  C.
C. 
�ڴ���Һ�з��������Cu2O�������ķ������з���Ҳ�ɷ���Cu2O����_________
��Cu2O����ķ�����_________________
��5��Ϊ�ⶨ��Ʒ��Cu2O�ĺ�������ȡ3.960g��Ʒ����ƿ�У�����30mL�����ữ��Fe2(SO4)3��Һ������������ַ�Ӧ����0.2000 mol��L��1��KMnO4��Һ�ζ����ظ�2��3�Σ�ƽ������KMnO4��Һ50.00mL��
�ٲ�Ʒ��Cu2O����������Ϊ_______
�����������ⶨ�������ƫ�ߵ�ԭ����_____
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com