
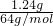 =0.02mol��n��B2O3��=0.01mol��
=0.02mol��n��B2O3��=0.01mol��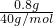 =0.02 mol��
=0.02 mol��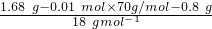 =0.01mol��
=0.01mol��

 100�ִ�����ĩ���ϵ�д�
100�ִ�����ĩ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�



| ��O3 | ��Zn��H2O |




�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


 ������ú�����У�����ԭ�Ӿ�����ͬһƽ���ڣ���1mol E ����ܺ�
������ú�����У�����ԭ�Ӿ�����ͬһƽ���ڣ���1mol E ����ܺ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�꽭��ʡ�����и�����һѧ�ڼ���Ծ���ѧ�Ծ��������棩 ���ͣ�ʵ����
��þ�����������Σ��������Ʊ����ᣨH3BO3����MgO���������£���þ�����(NH4)2SO4��Һ��Ϻ���ȣ���Ӧ����H3BO3�����MgSO4��Һ��ͬʱ�ų�NH3������MgSO4��Һ��ͨ��NH3��CO2���õ�MgCO3��������Һ��������ϴ�ӡ����պ��MgO����Һ��ѭ��ʹ�á��ش��������⣺
��1������������ƣ������νṹҲ�Ƚϸ��ӣ���Ӳ���ʯ��ѧʽΪCa2B6O11��5H2O�������дΪ���������ʽ ��
��2�������Ʊ������У��������ϴ���Ƿ���ȫ�ķ����� ��
��3��д��MgSO4��Һ��ͨ��NH3��CO2��Ӧ�Ļ�ѧ����ʽ ��
��4����ȷ��ȡ1.68 g��þ����ȫ��Ӧ���H3BO3����1.24 g��MgO 0.8 g������������ε���ɡ���д��������̣�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com