
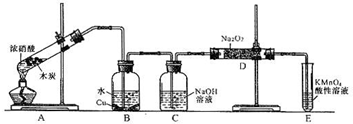
£®12Ј÷£©ƒ≥їѓ—І–Ћ»§–°„й“‘ћЉЇЌ≈®ѕхЋбќ™∆р Љ‘≠Ѕѕ£ђѕлјы”√“ї¬»їѓµ™”лєэ—хїѓƒ∆Јі”¶÷∆±Є—«ѕхЋбƒ∆°£…иЉ∆„∞÷√»зѕ¬£Ї£®Їц¬‘„∞÷√÷–њ’∆шµƒ”∞ѕм£©

«лїЎірѕ¬Ѕ–ќ ћв£Ї
£®1£©„∞÷√Aµƒ ‘є№÷–ЈҐ…ъЈі”¶µƒїѓ—ІЈљ≥ћ љ « °£
£®2£©≤¬≤вB÷–њ…“‘єџ≤мµƒ÷ч“™ѕ÷ѕу «
°£C∆њ≥цјіµƒ∆шће « °£
£®3£©“—÷™£ЇҐў “ќ¬ѕ¬іж‘ЏЈі”¶3HNO2=HNO3+2NO°ь+H2£їҐЏ‘ЏЋб–‘»№“Ї÷–£ђNO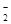 њ…љЂMnO
њ…љЂMnO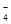 їє‘≠ќ™Mn2+«“ќё∆шће…ъ≥…°£–і≥цЉм—йD÷–≤ъќп «—«ѕхЋбƒ∆µƒЈљЈ®£Ї
їє‘≠ќ™Mn2+«“ќё∆шће…ъ≥…°£–і≥цЉм—йD÷–≤ъќп «—«ѕхЋбƒ∆µƒЈљЈ®£Ї
°£
£®4£©E„∞÷√µƒ„ч”√ « °£
£®5£©Ќђ—І√«Њ≠єэћ÷¬џ»ѕќ™…ѕ ц„∞÷√»‘”–»±ѕЁ£ђќ™±№√вDє№÷–…ъ≥…NaOH£ђƒг»ѕќ™”¶Є√љш––µƒЄƒљш « °£
£®1£©C+4HNO3£®≈®£©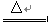 CO2°ь+4NO2°ь+2H2O
CO2°ь+4NO2°ь+2H2O
£®2£©»№“Ї÷рљ•±дјґ£їNO£®їт“ї—хїѓµ™£©£ї
£®3£©ЈљЈ®I£ЇљЂ…ъ≥…ќп÷√”Џ ‘є№÷–£ђЉ”»лѕ°ЅтЋб£ђ»ф≤ъ…ъќё…Ђ∆шће≤Ґ‘Џ“Ї√ж…ѕЈљ±дќ™Їм„Ў…Ђ£ђ‘тD÷–≤ъќп «—«ѕхЋбƒ∆°£Јі”¶µƒјл„”Јљ≥ћ љ «3NO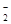 +2H+=NO
+2H+=NO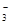 +2NO°ь+H2O
+2NO°ь+H2O
ЈљЈ®II£ЇљЂ…ъ≥…ќп÷√”Џ ‘є№÷–£ђЉ”»лЋб–‘KMnO4»№“Ї£ђ»ф»№“Ї„ѕ…ЂЌ »•£ђ‘тD÷–≤ъќп «—«ѕхЋбƒ∆£ђЈі”¶µƒјлЈљ≥ћ љ «5 NO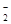 +2MnO
+2MnO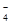 +6H+=5 NO
+6H+=5 NO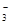 +2Mn2++3H2o£®∆дЋьЇѕјнір∞ЄЊщµ√Ј÷£©
+2Mn2++3H2o£®∆дЋьЇѕјнір∞ЄЊщµ√Ј÷£©
£®4£©ќь ’D÷–ќіЈі”¶ЌкµƒNO£ђ“‘√вќџ»Њњ’∆ш£®∆дЋьЇѕјнір∞ЄЊщµ√Ј÷£©
£®5£©‘ЏC°ҐD÷ЃЉд‘цЉ”“їЄц Ґ≈®ЅтЋбµƒѕі∆ш∆њ£®їт„∞ќёЋЃCaCl2µƒЄ…‘пє№£©£®∆дЋьЇѕјнір∞ЄЊщµ√Ј÷£©
°Њљвќц°њ


 √ы≈∆÷–—Іњќ ±„ч“µѕµЅ–ір∞Є
√ы≈∆÷–—Іњќ ±„ч“µѕµЅ–ір∞Є √чћмљћ”эњќ ±ћЎ—µѕµЅ–ір∞Є
√чћмљћ”эњќ ±ћЎ—µѕµЅ–ір∞Є ’гљ≠–¬њќ≥ћ»эќђƒњ±к≤в∆јњќ ±ћЎ—µѕµЅ–ір∞Є
’гљ≠–¬њќ≥ћ»эќђƒњ±к≤в∆јњќ ±ћЎ—µѕµЅ–ір∞Є ÷№÷№«еЉм≤вѕµЅ–ір∞Є
÷№÷№«еЉм≤вѕµЅ–ір∞Є
| ƒкЉґ | Єя÷–њќ≥ћ | ƒкЉґ | ≥х÷–њќ≥ћ |
| Єя“ї | Єя“ї√вЈ—њќ≥ћЌ∆Љц£° | ≥х“ї | ≥х“ї√вЈ—њќ≥ћЌ∆Љц£° |
| Єяґю | Єяґю√вЈ—њќ≥ћЌ∆Љц£° | ≥хґю | ≥хґю√вЈ—њќ≥ћЌ∆Љц£° |
| Єя»э | Єя»э√вЈ—њќ≥ћЌ∆Љц£° | ≥х»э | ≥х»э√вЈ—њќ≥ћЌ∆Љц£° |
њ∆ƒњ£ЇЄя÷–їѓ—І јі‘і£Ї ћв–Ќ£Ї‘ƒґЅјнљв
| ||
| ||
≤йњіір∞ЄЇЌљвќц>>
њ∆ƒњ£ЇЄя÷–їѓ—І јі‘і£Ї ћв–Ќ£Ї‘ƒґЅјнљв
≤йњіір∞ЄЇЌљвќц>>
њ∆ƒњ£ЇЄя÷–їѓ—І јі‘і£Ї ћв–Ќ£Ї
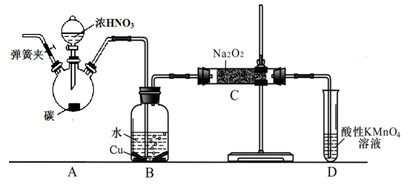
£®12Ј÷£©ƒ≥їѓ—І–Ћ»§–°„й“‘ћЉЇЌ≈®ѕхЋбќ™∆р Љ‘≠Ѕѕ£ђѕлјы”√“ї¬»їѓµ™”лєэ—хїѓƒ∆Јі”¶÷∆±Є—«ѕхЋбƒ∆°£…иЉ∆„∞÷√»зѕ¬£Ї£®Їц¬‘„∞÷√÷–њ’∆шµƒ”∞ѕм£©
«лїЎірѕ¬Ѕ–ќ ћв£Ї
£®1£©„∞÷√Aµƒ ‘є№÷–ЈҐ…ъЈі”¶µƒїѓ—ІЈљ≥ћ љ « °£
£®2£©≤¬≤вB÷–њ…“‘єџ≤мµƒ÷ч“™ѕ÷ѕу «
°£C∆њ≥цјіµƒ∆шће « °£
£®3£©“—÷™£ЇҐў “ќ¬ѕ¬іж‘ЏЈі”¶3HNO2=HNO3+2NO°ь+H2£їҐЏ‘ЏЋб–‘»№“Ї÷–£ђNO![]() њ…љЂMnO
њ…љЂMnO![]() їє‘≠ќ™Mn2+«“ќё∆шће…ъ≥…°£–і≥цЉм—йD÷–≤ъќп «—«ѕхЋбƒ∆µƒЈљЈ®£Ї
їє‘≠ќ™Mn2+«“ќё∆шће…ъ≥…°£–і≥цЉм—йD÷–≤ъќп «—«ѕхЋбƒ∆µƒЈљЈ®£Ї
°£
£®4£©E„∞÷√µƒ„ч”√ « °£
£®5£©Ќђ—І√«Њ≠єэћ÷¬џ»ѕќ™…ѕ ц„∞÷√»‘”–»±ѕЁ£ђќ™±№√вDє№÷–…ъ≥…NaOH£ђƒг»ѕќ™”¶Є√љш––µƒЄƒљш « °£
≤йњіір∞ЄЇЌљвќц>>
њ∆ƒњ£ЇЄя÷–їѓ—І јі‘і£Ї2013-2014—Іƒкљ≠ќч °Љ™∞≤ –Єя»э…ѕ—І∆Џ∆Џƒ©љћ—І÷ Ѕњ∆јЉџїѓ—І ‘Њн£®љвќц∞ж£© ћв–Ќ£Ї µ—йћв
—«ѕхЋбƒ∆єгЈЇ”√”Џ”°»Њ°Ґ∆ѓ∞„µ»––“µ£ђ‘Џљ®÷ю––“µ”√„чЈјґ≥ЉЅ£ђ‘Џ ≥∆Јє§“µ„чЈјЄѓЉЅЇЌ‘ц…ЂЉЅ£ђЋь «“ї÷÷«±‘Џ÷¬∞©ќп÷ £ђєэЅњїт≥§∆Џ ≥”√ґ‘»Ћ≤ъ…ъќ£Ї¶£ђ”…”Џ—«ѕхЋбƒ∆”–ѕћќґ£ђЌвєџ”лNaClѕаЋ∆£ђ‘шґаіќЈҐ…ъ±їµ± ≥—ќќу ≥µƒ ¬Љю°£ƒ≥їѓ—І–Ћ»§–°„й“‘ћЉЇЌ≈®ѕхЋбќ™∆р Љ‘≠Ѕѕ£ђ…иЉ∆»зѕ¬„∞÷√£ђјы”√“ї—хїѓµ™”лєэ—хїѓƒ∆Јі”¶÷∆±Є—«ѕхЋбƒ∆°££®Љ–≥÷„∞÷√ЇЌA÷–Љ”»»„∞÷√“—¬‘£ђ∆ш√№–‘“—Љм—й£©£ђ

≤й‘ƒ„ Ѕѕ£ЇҐўHNO2ќ™»хЋб£ђ “ќ¬ѕ¬іж‘ЏЈі”¶3HNO2£љHNO3£Ђ2NO°ь£ЂH2O£ї
ҐЏNO2£≠ƒ№±їЇ№ґа≥£Љыµƒ«њ—хїѓЉЅ—хїѓ£ђ»з‘ЏЋб–‘»№“Ї÷–њ…љЂMnO4£≠їє‘≠ќ™Mn2£Ђ«“ќё∆шће…ъ≥…°£
ҐџNO≤ї”лЉоЈі”¶£ђњ…±їЋб–‘KMnO4»№“Ї—хїѓќ™ѕхЋб
Ґ№HNO2‘ЏЋб–‘»№“Ї“≤ «“ї÷÷—хїѓЉЅ£ђ»зƒ№∞—I£≠—хїѓ≥…I2°£
ҐЁAgNO2 «“ї÷÷ƒ—»№”ЏЋЃ“„»№”ЏЋбµƒ∞„…ЂїѓЇѕќп°£
£®1£©–і≥цC÷–÷∆±Є—«ѕхЋбƒ∆Јі”¶µƒїѓ—ІЈљ≥ћ љ(Јі”¶ќпµƒќп÷ µƒЅњ÷Ѓ±»ќ™2£Ї1)??????? °£
£®2£©D„∞÷√µƒ„ч”√ «??????? °£
£®3£©Њ≠Љм—йC≤ъќп÷–—«ѕхЋбƒ∆ЇђЅњљѕ…ў°£Љ„Ќђ—І»ѕќ™C÷–≤ъќп≤їљц”–—«ѕхЋбƒ∆£ђїє”–ћЉЋбƒ∆ЇЌ«в—хїѓƒ∆°£ќ™≈≈≥эЄ…»≈Љ„Ќђ—І‘ЏB°ҐC„∞÷√Љд‘цЉ”„∞÷√E£ђE÷– ҐЈ≈µƒ ‘ЉЅ”¶ «________£®–і√ы≥∆£©°£
£®4£©»Ћће’э≥£µƒ—™Їмµ∞∞„÷–ЇђFe2+°£»фќу ≥—«ѕхЋб—ќ£®»зNaNO2£©£ђ‘тµЉ÷¬—™Їмµ∞∞„÷–Fe2+„™їѓќ™Fe3+ґш÷–ґЊ,Јю”√ќђ…ъЋЎCњ…љвґЊ°£ѕ¬Ѕ––р ц≤ї’э»Јµƒ «?????? °£
A£Ѓ—«ѕхЋб—ќ «їє‘≠ЉЅ????????????? ????????????? ????????????? ? B£Ѓќђ…ъЋЎC «їє‘≠ЉЅ
C£Ѓќђ…ъЋЎCљЂFe3+їє‘≠ќ™Fe2+????????????? ????????????? ????????????? ? D£Ѓ—«ѕхЋб—ќ±їїє‘≠
£®5£©ƒ№”√јіЉш±рNaNO2ЇЌNaClµƒЈљЈ® «?????????
A £Ѓ≤в»№“ЇpHЈ®
B£ЃЉ”ЋбЈ®
C£ЃAgNO3ЇЌHNO3Ѕљ÷÷ ‘ЉЅЈ®??
D£ЃЉ”KIµнЈџ£®Ћб–‘£©Ј®
E£Ѓ“‘…ѕЋƒ÷÷ЈљЈ®ґЉњ…
£®6£©ƒ≥Ќђ—І∞—деЋЃЉ”µљNaNO2»№“Їєџ≤мµљдеЋЃЌ …Ђ£ђ«л–і≥ціЋЈі”¶µƒјл„”Јљ≥ћ љ??????????????????????????????????????????? °£
≤йњіір∞ЄЇЌљвќц>>
∞ўґ»÷¬–≈ - ЅЈѕ∞≤бЅ–±н - ‘ћвЅ–±н
Їю±± °ї•Ѕ™Ќшќ•Ј®ЇЌ≤їЅЉ–≈ѕҐЊў±®∆љћ® | Ќш…ѕ”–Ї¶–≈ѕҐЊў±®„®«ш | µз–≈’©∆≠Њў±®„®«ш | …жјъ Ј–йќё÷ч“е”–Ї¶–≈ѕҐЊў±®„®«ш | …ж∆у«÷»®Њў±®„®«ш
ќ•Ј®ЇЌ≤їЅЉ–≈ѕҐЊў±®µзї∞£Ї027-86699610 Њў±®” ѕд£Ї58377363@163.com