
ФГЮоЩЋШмвКжажЛКЌгаЯТСа8жжРызгжаЕФФГМИжжЃК Mg2ЃЋЁЂHЃЋЁЂAgЃЋЁЂNaЃЋЁЂClЃЁЂHCO3ЃЁЂOHЃЁЂNO3ЃЃЌвбжЊИУШмвКгыТСЗДгІЗХГіЧтЦјЁЃЪдЛиД№ЯТСаЮЪЬтЃК
ЃЈ1ЃЉШєЗДгІКѓЩњГЩAl3ЃЋЃЌдђШмвКжаПЩФмДцдкЕФРызгга_______________ЃЌвЛЖЈДцдкЕФРызгга________________ЃЌвЛЖЈВЛДцдкЕФРызгга_________________ЗДгІЕФРызгЗНГЬЪНЮЊ________________ЁЃ
ЃЈ2ЃЉШєЗДгІКѓЩњГЩЁВAl(OH)4ЁГЃРызгЃЌдђШмвКжаПЩФмДцдкЕФРызгга_______________ЃЌвЛЖЈДцдкЕФРызгга________________ЃЌвЛЖЈВЛДцдкЕФРызгга_________________ЗДгІЕФРызгЗНГЬЪНЮЊ________________ЁЃ
ЃЈ1ЃЉMg2ЃЋЃЌNaЃЋЃЛ HЃЋЃЌClЃЃЛ AgЃЋЃЌ HCO3Ѓ ЃЌ OHЃЃЌNO3Ѓ ЃЛ2AlЃЋ 6HЃЋ ЃН 2Al3ЃЋ ЃЋ3H2Ёќ
ЃЈ2ЃЉClЃЃЌ NO3ЃЃЛ NaЃЋ ЃЌ OHЃЃЛ Mg2ЃЋЃЌ HЃЋЃЌAgЃЋЃЌ HCO3Ѓ
2AlЃЋ 2OHЃ ЃЋ 6H2OЃН 2ЁВAl(OH)4ЁГЃ ЃЋ3H2Ёќ
ЁОНтЮіЁП
ЪдЬтЗжЮіЃКЃЈ1ЃЉШєЗДгІКѓЩњГЩAl3ЃЋЃЌетЫЕУїИУШмвКвЛЖЈЯдЫсадЃЌЫљвдвЛЖЈДцдкЕФРызггаHЃЋЃЌЖјHCO3ЃЃЌ OHЃвЛЖЈЪЧВЛДцдкЕФЁЃгжвђЮЊдкЫсадЬѕМўЯТЃЌNO3ЃОпгаЧПбѕЛЏадЃЌЫфШЛФмЙЛШмНтЕЅжЪТСЃЌЕЋВЛФмЩњВњЧтЦјЃЌЫљвдNO3ЃвЛЖЈВЛДцдкЁЃгЩгкШмвКжаВЛПЩФмжЛгабєРызгЃЌЖјУЛгавѕРызгЃЌЫљвдТШРызгБиаыДцдкЃЌдђAgЃЋОЭвЛЖЈВЛФмДѓСПДцдкЁЃЫљвдвЛЖЈДцдкЕФРызггаHЃЋЃЌClЃЃЌПЩФмДцдкЕФРызгЪЧMg2ЃЋЁЂNaЃЋЃЌгаЙиЗДгІЕФЛЏбЇЗНГЬЪНЪЧ2AlЃЋ 6HЃЋ ЃН 2Al3ЃЋ ЃЋ3H2ЁќЁЃ
ЃЈ2ЃЉШєЗДгІКѓЩњГЩЁВAl(OH)4ЁГЃРызгЃЌетЫЕУїИУШмвКвЛЖЈЯдМюадЃЌЫљвдвЛЖЈДцдкЕФРызггаOHЃЃЌЖјHCO3ЃЁЂ Mg2ЃЋЁЂHЃЋЁЂAgЃЋЁЂвЛЖЈЪЧВЛДцдкЕФЁЃЭЌбљШмвКжаВЛПЩФмжЛгавѕРызгЃЌЖјУЛгабєРызгЃЌЫљвдФЦРызгБиаыДцдкЁЃЕЋClЃЁЂNO3ЃдђВЛФмШЗЖЈЃЌЫљвдвЛЖЈДцдкЕФРызггаNaЃЋЁЂOHЃЃЌгаЙиЕФЗНГЬЪНЪЧ2AlЃЋ 2OHЃ ЃЋ 6H2OЃН 2ЁВAl(OH)4ЁГЃ ЃЋ3H2ЁќЁЃ
ПМЕуЃКПМВщРызгДѓСПЙВДцЕФгаЙиХаЖЯ
ЕуЦРЃКБОЬтвВЪЧГЃМћЕФИпПМЬтаЭЃЌЪєгкжаЕШФбЖШЕФЪдЬтЃЌЖдбЇЩњЕФФмСІЬсГіСЫИќИпЕФвЊЧѓЁЃБОЬтМШПЩвдЙЎЙЬбЇЩњЖдГЃМћРызгФмЗёДѓСПЙВДцЕФМьбщЃЌгжгаРћгкХрбјбЇЩњЫМЮЌЕФбЯНїадЁЂПЦбЇадвдМАНтОіЪЕМЪЮЪЬтЕФФмСІЃЌЪЧИпПМЪдЬтжаЕФИпЦЕЕуЁЃ


 53ЫцЬУВтЯЕСаД№АИ
53ЫцЬУВтЯЕСаД№АИ
| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃКдФЖСРэНт
| X |
| X |
| H2O |
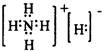
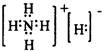
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
 ФГЮоЩЋЯЁШмвКXжаЃЌПЩФмКЌгаЯТБэЫљСаРызгжаЕФФГМИжжЃЎ
ФГЮоЩЋЯЁШмвКXжаЃЌПЩФмКЌгаЯТБэЫљСаРызгжаЕФФГМИжжЃЎ| вѕРызг | CO32-ЁЂSiO32-ЁЂAlO2-ЁЂCl- |
| бєРызг | Al3+ЁЂCu2+ЁЂMg2+ЁЂNH4+ЁЂNa+ |
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
 ЃЈ IЃЉФГЮоЩЋЯЁШмвКXжаЃЌПЩФмКЌгаЯТБэЫљСаРызгжаЕФФГМИжжЃЎ
ЃЈ IЃЉФГЮоЩЋЯЁШмвКXжаЃЌПЩФмКЌгаЯТБэЫљСаРызгжаЕФФГМИжжЃЎ| вѕРызг | CO32-ЁЂSiO32-ЁЂAlO2-ЁЂCl- |
| бєРызг | Al3+ЁЂCu2+ЁЂMg2+ЁЂNH4+ЁЂNa+ |
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
A.гУЪЏФЋзіЕчМЋЕчНтТШЛЏяЇХЈШмвКЃЌзюЖржЛФмЕУЕН2жжЦјЬх
B.БъзМзДПіЯТЃЌНЋ2 gТСЦЌЭЖШыЕН20 mL18 molЃЏLЕФСђЫсжажЦШЁЧтЦј
C.ЯђФГЮоЩЋШмвКжаЕЮМгBa(NO3)2ШмвКЃЌШєЩњГЩАзЩЋГСЕэЃЌдйМгЯЁбЮЫсГСЕэВЛЯћЪЇЃЌдђПЩШЗЖЈИУШмвКжавЛЖЈКЌга![]()
D.ВЛгУЦфЫћЪдМСЃЌжЛРћгУвКЬхМфЕФСНСНЛьКЯОЭФмМјБ№ОЦОЋЁЂЫФТШЛЏЬМЁЂМзБНЁЂфхЫЎКЭNaHCO3ШмвК
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2013-2014бЇФъЩТЮїЪЁИпШ§ЩЯбЇЦкЕкЖўДЮдТПМРэзлЛЏбЇЪдОэЃЈНтЮіАцЃЉ ЬтаЭЃКбЁдёЬт
ЃЈ1ЃЉЮ§ЮЊЕкЂєAзхдЊЫиЃЌЮ§ЕФЕЅжЪКЭЛЏКЯЮягыФГаЉЮяжЪЕФЛЏбЇаджЪгааэЖрЯрЫЦжЎДІЁЃвбжЊЮ§дЊЫиОпгаШчЯТаджЪЃК
 ЃЛ
ЃЛ




 ЁЃ
ЁЃ
ЪдЛиД№ЃК
ЂйЮ§ШмгкбЮЫсЃЌдйЯђЗДгІКѓЕФШмвКжаЭЈШыТШЦјЃЌгаЙиЗДгІРрЫЦгкЬњЕФЯргІБфЛЏЃЌЪдаДГігаЙиЗДгІЕФЛЏбЇЗНГЬЪНЃК_____________________________________________________ЃЌ_______________________________________ЁЃ
ЂкНЋЂйжаШмвКеєИЩКѓМЬајзЦЩеЫљЕУЙЬЬхЃЌБфЛЏЙ§ГЬРрЫЦгкFeCl3ШмвКЯргІЕФБфЛЏЃЌдђзюКѓЕУЕНЕФЙЬЬхЮяжЪЪЧ__________ЃЈЬюЛЏбЇЪНЃЉЁЃ
ЂлШєПЩгУSnCl2ШмвКгыЙ§СПЕФМюШмвКЗДгІЕФЗНЗЈжЦSnЃЈOHЃЉ2ЃЌИУМюПЩбЁгУ________ЁЃ
ЃЈ2ЃЉФГЮоЩЋЯЁШмвКXжаЃЌПЩФмКЌгаЯТБэЫљСаРызгжаЕФФГМИжжЁЃ
|
вѕРызг |
|
|
бєРызг |
|
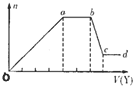
ЯжШЁИУШмвКЪЪСПЃЌЯђЦфжаМгШыФГЪдМСYЃЌВњЩњГСЕэЕФЮяжЪЕФСПЃЈnЃЉгыМгШыЪдМСYЬхЛ§ЃЈVЃЉЕФЙиЯЕШчЭМЫљЪОЁЃ

ЂйШєYЪЧбЮЫсЃЌдђШмвКжаКЌгаЕФН№ЪєбєРызгЪЧ_________ЃЌabЖЮЗЂЩњЗДгІЕФРызгЗНГЬЪНЮЊ_______________ЃЌЭМжаoaЖЮВЮМгЗДгІЕФвѕРызгЕФЮяжЪЕФСПжЎБШЮЊ___________________ЁЃ
ЂкШєYЪЧNaOHШмвКЃЌдђbcЖЮЗДгІЕФРызгЗНГЬЪНЮЊ____________________ЁЃ
ШєВЛПМТЧРызгЕФЫЎНтЃЌКіТдHЃЋКЭOHЃЕФгАЯьЃЌЧвШмвКжажЛДцдкЫФжжРызгЃЌдђЫќУЧЕФРызгИіЪ§БШЮЊ_____________________________________________ЃЈАДбєРызгдкЧАЃЌвѕРызгдкКѓЃЌИпМлдкЧАЃЌЕЭМлдкКѓЕФЫГађХХСаЃЉЁЃ
ВщПДД№АИКЭНтЮі>>
АйЖШжТаХ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com