
(14·Ц)
ДіСРѕїРЎЧйОЄМЅѕїИхЛбРФМхјюПВМъ·ўЙъµз»ЇС§ёЇКґАаРНµДУ°ПмТтЛШЈ¬Ѕ«»мєПѕщФИµДРВЦЖМъ·ЫєНМј·ЫЦГУЪЧ¶РОЖїµЧІїЈ¬ИыЙПЖїИы(ИзНј1)ЎЈґУЅєН·µО№ЬЦРµОИлјёµОґЧЛбИЬТєЈ¬Н¬К±ІвБїИЭЖчЦРµДС№Зї±д»ЇЎЈ
ЈЁ1Ј©ЗлНкіЙТФПВКµСйЙијЖ±н(±нЦРІ»ТЄБфїХёс)Јє
| ±аєЕ | КµСйДїµД | Мј·Ы/g | Мъ·Ы/g | ґЧЛб/% |
| ўЩ | ОЄТФПВКµСйЧчІОХХ | 0.5 | 2.0 | 90.0 |
| ўЪ | ґЧЛбЕЁ¶ИµДУ°Пм | 0.5 | | 36.0 |
| ўЫ | | 0.2 | 2.0 | 90.0 |

| КµСйІЅЦиєНЅбВЫ(І»ТЄЗуРґѕЯМеІЩЧч№эіМ)Јє |
ЈЁ1Ј©ўЪ2.0 ўЫМј·Ыє¬БїµДУ°Пм
ЈЁ2Ј©ОьСхёЇКґ 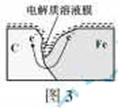 »№Ф·ґУ¦ 2H2O+O2+4e-=4OH- ЈЁ»т4H++O2+4e-=2H2OЈ©
»№Ф·ґУ¦ 2H2O+O2+4e-=4OH- ЈЁ»т4H++O2+4e-=2H2OЈ©
ЈЁ3Ј©·ґУ¦·ЕИИЈ¬ОВ¶ИЙэёЯЈ¬Ме»эЕтХН
ЈЁ4Ј©КµСйІЅЦиєНЅбВЫЈЁІ»ТЄЗуРґѕЯМеІЩЧч№эіМЈ©
ўЩТ©Ж·УГБїєНІЩЧчН¬±аєЕўЩКµСйЈЁ¶аїЧПрЖ¤ИыФцјУЅшЎўіцµј№ЬЈ©
ўЪНЁИллІЖшЕЕѕ»ЖїДЪїХЖшЈ»
ўЫµОИлґЧЛбИЬТєЈ¬Н¬К±ІвБїЖїДЪС№Зї±д»ЇЈЁТІїЙІвОВ¶И±д»ЇЈ¬јмСйFe2+µИЈ©ЎЈ
Из№ыЖїДЪС№ЗїФцґуЈ¬јЩЙиТ»іЙБўЎЈ·сФтјЩЙиТ»І»іЙБўЎЈ
ЈЁ±ѕМвКфУЪїЄ·ЕРФКФМвЈ¬єПАнґр°ёѕщёш·ЦЈ©
ЅвОцКФМв·ЦОцЈєЈЁ1Ј©МЅѕїУ°Пм»ЇС§·ґУ¦ЛЩВКЈ¬ГїґОЦ»ДЬёД±дТ»ёц±дБїЈ¬№КУРўЪЦРМъµДБїІ»±дЈ¬ОЄ2.0gЈ»ўЫЦРёД±дБЛМј·ЫµДЦКБїЈ¬№КОЄМЅѕїМј·ЫµДБї¶ФЛЩВКµДУ°ПмЎЈ
ЈЁ2Ј©С№ЗїУлЖшМеµДОпЦКµДБїіЙХэ±ИЈ¬ґУНјЦРїЙТФїґіцЈ¬ЖшМеµДБїїЄКјФцјУЈ¬єујхЙЩЈ¬№КОЄОьСхёЇКґЈ»»оЖГЅрКфЧцёєј«Ј¬№КМјОЄХэј«Ј¬·ўЙъ»№Ф·ґУ¦ЎЈ
ЈЁ3Ј©ґУМе»эµДУ°ПмТтЛШЧЕКЦЈ¬ОВ¶ИЙэёЯЈ¬Ме»эФцґу
ЈЁ4Ј©»щУЪјЩЙиТ»Ј¬їЙЦЄЈ¬ІъЙъЗвЖшЈ¬·ўЛНДЗР©±д»ЇЈ¬ґУ±д»ЇИлКЦїјВЗ
їјµгЈєКµСйЙијЖУлМЅѕїЈ¬У°Пм»ЇС§·ґУ¦ЛЩВКµДТтЛШЎЈ


 РЗј¶їЪЛгМмМмБ·ПµБРґр°ё
РЗј¶їЪЛгМмМмБ·ПµБРґр°ё Гў№ыЅМёЁґп±кІвКФѕнПµБРґр°ё
Гў№ыЅМёЁґп±кІвКФѕнПµБРґр°ё
| Дкј¶ | ёЯЦРїОіМ | Дкј¶ | іхЦРїОіМ |
| ёЯТ» | ёЯТ»Гв·СїОіМНЖјцЈЎ | іхТ» | іхТ»Гв·СїОіМНЖјцЈЎ |
| ёЯ¶ю | ёЯ¶юГв·СїОіМНЖјцЈЎ | іх¶ю | іх¶юГв·СїОіМНЖјцЈЎ |
| ёЯИэ | ёЯИэГв·СїОіМНЖјцЈЎ | іхИэ | іхИэГв·СїОіМНЖјцЈЎ |
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈєµҐСЎМв
ПВНјЛщКѕµДКµСйЈ¬ДЬґпµЅКµСйДїµДµДКЗ
| A | B | C | D |
 |  |  |  |
| СйЦ¤»ЇС§ДЬЧЄ»ЇОЄµзДЬ | СйЦ¤ОВ¶И¶ФЖЅєвТЖ¶ЇµДУ°Пм | СйЦ¤Мъ·ўЙъОцЗвёЇКґ | СйЦ¤·ЗЅрКфРФClЈѕ C Јѕ Si |
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈєµҐСЎМв
ПВБРНјКѕКµСйєПАнµДКЗ
| AЈ®Нј1ОЄЦ¤Гч·ЗЅрКфРФЗїИхЈєS>C>Si | BЈ®Нј2ОЄЦЖ±ёЙЩБїСхЖш |
| CЈ®Нј3ОЄЕдЦЖТ»¶ЁЕЁ¶ИБтЛбИЬТє | DЈ®Нј4ЦЖ±ёІўКХјЇЙЩБїNO2ЖшМе |
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈєµҐСЎМв
ПВБРКµСйДЬґпµЅДїµДµДКЗ
| AЈ®УГРїУлПЎПхЛбЦЖ±ёH2 |
| BЈ®УГЛбРФKMnO4ИЬТєјш±р1-ОмП©єНјЧ±Ѕ |
| CЈ®УГ¶Ўґп¶ыР§У¦јш±рј¦µ°°ЧИЬТєєНКіСОЛ® |
| DЈ®УГЛ®АґіэИҐNO2ЦРЙЩБїµДNO |
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈєМоїХМв
ёхМъїуµДЦчТЄіЙ·ЦїЙ±нКѕОЄFeOЎ¤Cr2O3Ј¬»№є¬УРMgOЎўAl2O3ЎўFe2O3µИФУЦКЈ¬ТФПВКЗТФёхМъїуОЄФБПЦЖ±ёЦШёхЛбјШЈЁK2Cr2O7Ј©µДБчіМНјЈє
ТСЦЄЈєўЩ4FeOЎ¤Cr2O3+ 8Na2CO3+ 7O2 8Na2CrO4 + 2 Fe2O3 + 8CO2ЎьЈ»
8Na2CrO4 + 2 Fe2O3 + 8CO2ЎьЈ»
ўЪNa2CO3 + Al2O3 2NaAlO2 + CO2ЎьЈ»ўЫCr2O72Ј+ H2O
2NaAlO2 + CO2ЎьЈ»ўЫCr2O72Ј+ H2O
 2CrO42Ј + 2H+
2CrO42Ј + 2H+
ёщѕЭМвТв»ШґрПВБРОКМвЈє
ЈЁ1Ј©№ММеXЦРЦчТЄє¬УР_________ЈЁМоРґ»ЇС§КЅЈ©Ј»ТЄјмІвЛб»ЇІЩЧчЦРИЬТєµДpHКЗ·сµИУЪ4.5Ј¬У¦ёГК№УГ__________ЈЁМоРґТЗЖч»тКФјБГыіЖЈ©ЎЈ
ЈЁ2Ј©Лб»ЇІЅЦиУГґЧЛбµчЅЪИЬТєpH<5Ј¬ЖдДїµДКЗ_________________________________ЎЈ
ЈЁ3Ј©ІЩЧчўуУР¶аІЅЧйіЙЈ¬»сµГK2Cr2O7ѕ§МеµДІЩЧчТАґОКЗЈєјУИлKCl№ММеЎўХф·ўЕЁЛхЎў Ўў№эВЛЎў_______ЎўёЙФпЎЈ
ЈЁ4Ј©ПВ±нКЗПа№ШОпЦКµДИЬЅв¶ИКэѕЭЈ¬ІЩЧчўу·ўЙъ·ґУ¦µД»ЇС§·ЅіМКЅКЗЈєNa2Cr2O7+2KCl ЎъK2Cr2O7Ўэ+2NaClЎЈ
| ОпЦК | ИЬЅв¶И/(g/100gЛ®) | ||
| 0ЎгC | 40ЎгC | 80ЎгC | |
| KCl | 28 | 40.1 | 51.3 |
| NaCl | 35.7 | 36.4 | 38 |
| K2Cr2O7 | 4.7 | 26.3 | 73 |
| Na2Cr2O7 | 163 | 215 | 376 |
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈєМоїХМв
»ФНїуКЇЦчТЄє¬УРБт»ЇСЗНЈЁCu2SЈ©ј°ЙЩБїВцКЇЈЁSiO2Ј©ЎЈТ»ЦЦТФ»ФНїуКЇОЄФБПЦЖ±ёПхЛбНµД№¤ТХБчіМИзПВЈє
ўЕРґіцЅюИЎ№эіМЦРCu2SИЬЅвµДАлЧУ·ЅіМКЅЈє____________________ЎЈ
ўЖ»ШКХS№эіМЦРОВ¶ИїШЦЖФЪ50Ў«60ЎжЦ®јдЈ¬І»ТЛ№эёЯ»т№эµНµДФТтКЗ_____________ЎЈ
ўЗЖшМеNOxУлСхЖш»мєПєуНЁИлЛ®ЦРДЬЙъіЙБчіМЦРїЙС»·АыУГµДТ»ЦЦОпЦКЈ¬ёГ·ґУ¦µД»ЇС§·ЅіМКЅОЄ___________________________Ј»ПтВЛТєMЦРјУИлЈЁ»тНЁИлЈ©ПВБР__________ЈЁМоЧЦДёЈ©ОпЦКЈ¬µГµЅБнТ»ЦЦїЙС»·АыУГµДОпЦКЎЈ
aЈ®Мъ bЈ®ВИЖш cЈ®ёЯГМЛбјШ
ўИ±ЈОВіэМъ№эіМЦРЈ¬јУИлCuOµДДїµДКЗ________________________Ј»Хф·ўЕЁЛхК±Ј¬ТЄУГHNO3ИЬТєµчЅЪИЬТєµДpHЈ¬ЖдАнУЙКЗ___________________________ЎЈ
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈєјЖЛгМв
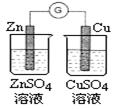
ЈЁ1Ј©ДіС§П°РЎЧйАыУГПВНјЧ°ЦГЦЖИЎВИЖшІўМЅѕїЖдРФЦКЎЈ
ўЩјЧЧ°ЦГЦР·ґУ¦µД»ЇС§·ЅіМКЅКЗ Ј»
ўЪЦ¤ГчТТЧ°ЦГЦРFeCl2ИЬТєУлCl2·ўЙъБЛ·ґУ¦µДКµСй·Ѕ·ЁКЗЈЁЦ»ЧўГчКФјБЎўПЦПуЈ© Ј»
ўЫ±ыЧ°ЦГЦРНЁИлЙЩБїCl2Ј¬їЙЦЖµГДіЦЦЙъ»оЦРіЈУГµДЖЇ°ЧЎўПы¶ѕµДОпЦКЎЈТСЦЄМјЛбµДЛбРФЗїУЪґОВИЛбЈ¬Фт±ыЦР·ґУ¦µД»ЇС§·ЅіМКЅКЗ ЎЈ
ЈЁ2Ј©УРТ»Жїі¤ЖЪ·ЕЦГµДЖЇ°Ч·ЫЈ¬ЗлАыУГТФПВТЗЖчєНКФјБЈ¬НкіЙёГЖЇ°Ч·ЫіЙ·ЭµДМЅѕїЎЈ
КФ№ЬЎўЅєН·µО№ЬЎўґшµј№ЬµДµҐїЧИыЎўХфБуЛ®Ўў1molЎ¤L-1СОЛбЎўЖ·ємИЬТєЎўРВЦЖіОЗеКЇ»ТЛ®ЎЈ
ЎѕМбіцјЩЙиЎїјЩЙиТ»ЈєёГЖЇ°Ч·ЫОґ±дЦКЈ¬є¬CaCl2ЎўCaЈЁClOЈ©2Ј»
јЩЙи¶юЈєёГЖЇ°Ч·ЫИ«Ії±дЦКЈ¬є¬ Ј»
јЩЙиИэЈєёГЖЇ°Ч·ЫІї·Ц±дЦКЈ¬є¬CaCl2ЎўCaЈЁClOЈ©2ЎўCaCO3 ЎЈ
ЎѕЅшРРКµСйЎїФЪґрМвїЁЙПНкіЙПВ±нЈЁІ»±ШјмСйCa2+ЎўCl-Ј©Јє
| КµСйІЅЦи | Ф¤ЖЪПЦПуєНЅбВЫ | |
| ўЩ | УГAКФ№ЬИЎЙЩБїіОЗеКЇ»ТЛ®±ёУГЈ¬УГBКФ№ЬИЎЙЩБїСщЖ·Ј¬ФЩПтBКФ№Ь | ИфОЮЖшМе·ЕіцЗТіОЗеКЇ»ТЛ®Оґјы»лЧЗЈ¬ФтјЩЙиТ»іЙБўЈ» |
| ўЪ | | |
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈєКµСйМв
ЈЁ14·ЦЈ©
Ді»ЇС§РЛИ¤РЎЧйОЄМЅѕїCl2ЎўBr2ЎўFe3+µДСх»ЇРФЗїИхЈ¬ЙијЖБЛИзПВКµСйЈє
ЈЁ1Ј© ўЩјмІйЖшМе·ўЙъЧ°ЦГAµДЖшГЬРФµДІЩЧчКЗЈєЈЯЈЯЈЯЈЯЈЯЈЯЈЯЈЯЈЯЈЯЈЯЈЯ
ўЪХыМЧКµСйЧ°ЦГґжФЪТ»ґ¦ГчПФІ»ЧгЈ¬ЗлЦёіцЈЯЈЯЈЯЈЯЈЯЈЯЈЯЈЯЈЯЈЯЈЯЈЯЈЯЈ®
ЈЁ2Ј© УГёДХэєуµДЧ°ЦГЅшРРКµСйЈ®КµСй№эіМИзПВЈє
| КµСйІЩЧч | КµСйПЦПу | ЅбВЫ |
| ґтїЄ»оИыaЈ¬ПтФІµЧЙХЖїЦРµОИлККБїЕЁСОЛбЈ»И»єу№Ш±Х»оИыaЈ¬µгИјѕЖѕ«µЖ | DЧ°ЦГЦРЈєИЬТє±дєм EЧ°ЦГЦРЈєЛ®ІгИЬТє±д»ЖХсµґєуCCl4ІгОЮГчПФ±д»Ї | Cl2ЎўBr2ЎўFe3+µДСх»ЇРФУЙЗїµЅИхµДЛіРтОЄЈє ______________________ |
| ўсЈ®ЈЁSCNЈ©2РФЦКУлВ±ЛШµҐЦКАаЛЖЈ®Сх»ЇРФЈєCl2ЈѕЈЁSCNЈ©2Ј® ўтЈ®Cl2єНBr2·ґУ¦ЙъіЙBrClЈ¬ЛьіКємЙ«ЈЁВФґш»ЖЙ«Ј©Ј¬·РµгОЄ5ЎжЈ¬УлЛ®·ўЙъЛ®Ѕв·ґУ¦Ј® ўуЈ®AgClOЎўAgBrOѕщїЙИЬУЪЛ®Ј® |
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈєКµСйМв
ДіН¬С§ФЪЖшМеМе»эІв¶ЁТЗЦРУГГѕєНБтЛб·ґУ¦Ів¶Ё1 molЗвЖшМе»эЎЈНкіЙПВБРМоїХЈє
ЈЁ1Ј©AЦР·ўЙъ·ґУ¦µДАлЧУ·ЅіМКЅОЄ______________________ЎЈ
ЈЁ2Ј©јмІйЧ°ЦГЖшГЬРФµД·Ѕ·ЁКЗИыЙПBЖїµДІЈБ§ИыЈ¬УГПрЖ¤ИыИыЅфAЖїјУБПїЪЈ¬µ±їґµЅ__________________ПЦПуК±Ј¬їЙТФИ·ИПЧ°ЦГЖшГЬРФБјєГЎЈ
ЈЁ3Ј©ТСЦЄТєМеБїЖїµДїМ¶И·¶О§КЗ110~130 mLЈ¬КµСйК±іЖИЎГѕґшµДЦКБїТЄїШЦЖФЪ0.100~0.110 gЦ®јдЈ¬ДїµДКЗ_________ЎЈ
ЈЁ4Ј©НкіЙТ»ґОІв¶ЁКµСйЈ¬РиТЄ2ґОУГЧўЙдЖчійЖшЈ¬ЖдЦРРиТЄјЗВјµДКЗµЪ__________ґОійіцЖшМеµДМе»эЎЈ
ЈЁ5Ј©ПВБРЗйїц»бµјЦВКµСйЅб№ыЖ«ёЯµДКЗ______ЈЁМо±аєЕЈ©
a. Гѕ±нГжµДСх»ЇД¤Г»УРіэѕЎ b. ґўТєЖїЦРµДТєМеКЗЛ®
c. ОґАдИґµЅКТОВѕН¶БКэ d. Ч°ЦГЖшГЬРФІ»єГ
Ійїґґр°ёєНЅвОц>>
°Щ¶ИЦВРЕ - Б·П°ІбБР±н - КФМвБР±н
єю±±КЎ»ҐБЄНшОҐ·ЁєНІ»БјРЕПўѕЩ±ЁЖЅМЁ | НшЙПУРє¦РЕПўѕЩ±ЁЧЁЗш | µзРЕХ©ЖѕЩ±ЁЧЁЗш | ЙжАъК·РйОЮЦчТеУРє¦РЕПўѕЩ±ЁЧЁЗш | ЙжЖуЗЦИЁѕЩ±ЁЧЁЗш
ОҐ·ЁєНІ»БјРЕПўѕЩ±Ёµз»°Јє027-86699610 ѕЩ±ЁУКПдЈє58377363@163.com