
”¾ĢāÄæ”æ°ĖÖÖ¶ĢÖÜĘŚŌŖĖŲx”¢y”¢z”¢d”¢e”¢f”¢g”¢hŌ×ÓŠņŹżŅĄ“ĪµŻŌö£¬ĘäŌ×Ó°ė¾¶”¢×īøßÕż¼Ū»ņ×īµĶøŗ¼ŪČēĻĀ±ķĖłŹ¾”£
x | y | z | d | e | f | g | h | |
Ō×Ó°ė¾¶/nm | 0.037 | 0.077 | 0.075 | 0.074 | 0.186 | 0.143 | 0.102 | 0.099 |
×īøßÕż»ÆŗĻ¼Ū»ņ×īµĶøŗ»ÆŗĻ¼Ū | +1 | +4 | +5 | -2 | +1 | +3 | -2 | -1 |
»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©±Č½Ļd”¢f³£¼ūĄė×ӵİė¾¶“󊔣ØÓĆ»ÆѧŹ½±ķŹ¾£¬ĻĀĶ¬£©£ŗ_______>________£»±Č½Ļg”¢hµÄ×īøß¼ŪŃõ»ÆĪļ¶ŌÓ¦Ė®»ÆĪļµÄĖįŠŌĒæČõ£ŗ________>__________”£
£Ø2£©ÓÉx”¢dĮ½ÖÖŌŖĖŲ×é³É18µē×ӵķÖ×Ó£¬Ęäµē×ÓŹ½ĪŖ____________£¬¾²ā¶ØĘäĪŖ¶žŌŖČõĖį£¬ĘäĖįŠŌ±ČĢ¼ĖįČõ£¬Š“³öĘ䵌Ņ»²½µēĄėµÄµēĄė·½³ĢŹ½____________”£
£Ø3£©y”¢d”¢e×é³ÉĪļÖŹe2yd3µÄĖ®ČÜŅŗ³Ź¼īŠŌ£¬ĘäŌŅņŹĒ____________________£ØÓĆĄė×Ó·½³ĢŹ½±ķŹ¾£©£¬µē½āøĆĪļÖŹe2yd3µÄČÜŅŗ£¬ŌĄķČēĶ¼ĖłŹ¾”£

ĘäÖŠ£¬Ąė×Ó½»»»Ä¤Ź¹ÓƵďĒ__________£ØĢī”°ŃōĄė×Ó½»»»Ä¤”±»ņ”°ŅõĄė×Ó½»»»Ä¤”±£©£¬Ņõ¼«²śÉśµÄĪļÖŹAµÄ»ÆѧŹ½ĪŖ____________”£
£Ø4£©fµÄ·ŪÄ©ŌŚ1000”ꏱæÉÓėN2·“Ó¦ÖʱøfN”£ŌŚfµÄ·ŪÄ©ÖŠĢķ¼ÓÉŁĮæNH4Cl¹ĢĢå²¢³ä·Ö»ģŗĻ£¬ÓŠĄūÓŚfNµÄÖʱø£¬ĘäÖ÷ŅŖŌŅņŹĒ____________________________________”£
”¾“š°ø”æO2£ Al3+ HClO4 H2SO4 ![]() H2O2
H2O2 ![]() H£«£«HO2£ CO32- +H2O
H£«£«HO2£ CO32- +H2O![]() HCO3- +OH- HCO3- +H2O
HCO3- +OH- HCO3- +H2O![]() H2CO3 +OH- ŃōĄė×Ó½»»»Ä¤ H2 NH4Cl·Ö½ā²śÉśµÄHClÄܹ»ĘĘ»µAl±ķĆęµÄAl2O3±”Ĥ
H2CO3 +OH- ŃōĄė×Ó½»»»Ä¤ H2 NH4Cl·Ö½ā²śÉśµÄHClÄܹ»ĘĘ»µAl±ķĆęµÄAl2O3±”Ĥ
”¾½āĪö”æ
°ĖÖÖ¶ĢÖÜĘŚŌŖĖŲx”¢y”¢z”¢d”¢e”¢f”¢g”¢hŌ×ÓŠņŹżŅĄ“ĪµŻŌö£¬½įŗĻŌ×Ó°ė¾¶”¢×īøßÕż¼Ū»ņ×īµĶøŗ¼ŪŹż¾ŻæÉµĆ£ŗxŹĒHŌŖĖŲ”¢yŹĒCŌŖĖŲ”¢zŹĒNŌŖĖŲ”¢dŹĒOŌŖĖŲ”¢eŹĒNaŌŖĖŲ”¢fŹĒAlŌŖĖŲ”¢gŹĒSŌŖĖŲ”¢hŹĒClŌŖĖŲ”£
£Ø1£©dŹĒOŌŖĖŲ”¢fŹĒAlŌŖĖŲ£¬O2-ÓėAl3+¾ßÓŠĻąĶ¬µē×Ó²ć½į¹¹£¬ŗĖµēŗÉŹżO2-Š”ÓŚAl3+£¬ĖłŅŌ°ė¾¶O2->Al3+£»gŹĒSŌŖĖŲ”¢hŹĒClŌŖĖŲ£¬·Ē½šŹōŠŌCl>S£¬ĖłŅŌ×īøß¼ŪŃõ»ÆĪļ¶ŌÓ¦Ė®»ÆĪļµÄĖįŠŌ£ŗHClO4>H2SO4”£
£Ø2£©xŹĒHŌŖĖŲ”¢dŹĒOŌŖĖŲ£¬×é³ÉµÄ18µē×Ó·Ö×ÓĪŖH2O2£¬ŹōÓŚ¹²¼Ū»ÆŗĻĪļ£¬µē×ÓŹ½ĪŖ£ŗ![]() £»ĘäĪŖ¶žŌŖČõĖį£¬Ōņ“ęŌŚµēĄėĘ½ŗā£¬µŚŅ»²½µēĄėÉś³ÉH+ŗĶHO2-£¬¹ŹµēĄė·½³ĢŹ½ĪŖ£ŗH2O2
£»ĘäĪŖ¶žŌŖČõĖį£¬Ōņ“ęŌŚµēĄėĘ½ŗā£¬µŚŅ»²½µēĄėÉś³ÉH+ŗĶHO2-£¬¹ŹµēĄė·½³ĢŹ½ĪŖ£ŗH2O2![]() H+£«HO2-”£
H+£«HO2-”£
£Ø3£©yŹĒCŌŖĖŲ”¢dŹĒOŌŖĖŲ”¢eŹĒNaŌŖĖŲ£¬e2yd3ĪŖNa2CO3£¬ŹōÓŚĒæ¼īČõĖįŃĪ£¬CO32-Ė®½āŹ¹ČÜŅŗ³Ź¼īŠŌ£¬Ąė×Ó·½³ĢŹ½ĪŖ£ŗCO32-+H2O![]() HCO3-+OH-”¢HCO3-+H2O
HCO3-+OH-”¢HCO3-+H2O![]() H2CO3+OH-£»Óɵē½āNa2CO3ČÜŅŗµÄŌĄķĶ¼æÉµĆ£¬×ó±ßĪŖŃō¼«£¬·¢ÉśŃõ»Æ·“Ӧɜ³ÉŃõĘų£¬ÓŅ±ßĪŖŅõ¼«£¬Ė®ÖŠ+1¼ŪH·¢Éś»¹Ō·“Ӧɜ³ÉĒāĘų£¬Ķ¬Ź±µĆµ½NaOHČÜŅŗ£¬ĖłŅŌĄė×Ó½»»»Ä¤ŌŹŠķNa+ĶعżŅĘĻņÓŅ±ß£¬×čÖ¹OH-Ļņ×óŅĘ¶Æ£¬¹ŹÓ¦ĪŖŃōĄė×Ó½»»»Ä¤£»Ņõ¼«²śÉśĒāĘų£¬»ÆѧŹ½ĪŖH2”£
H2CO3+OH-£»Óɵē½āNa2CO3ČÜŅŗµÄŌĄķĶ¼æÉµĆ£¬×ó±ßĪŖŃō¼«£¬·¢ÉśŃõ»Æ·“Ӧɜ³ÉŃõĘų£¬ÓŅ±ßĪŖŅõ¼«£¬Ė®ÖŠ+1¼ŪH·¢Éś»¹Ō·“Ӧɜ³ÉĒāĘų£¬Ķ¬Ź±µĆµ½NaOHČÜŅŗ£¬ĖłŅŌĄė×Ó½»»»Ä¤ŌŹŠķNa+ĶعżŅĘĻņÓŅ±ß£¬×čÖ¹OH-Ļņ×óŅĘ¶Æ£¬¹ŹÓ¦ĪŖŃōĄė×Ó½»»»Ä¤£»Ņõ¼«²śÉśĒāĘų£¬»ÆѧŹ½ĪŖH2”£
£Ø4£©fŹĒAlŌŖĖŲ£¬ĖłŅŌfNĪŖAlN£¬Alµ„ÖŹŹĒ»īĘĆ½šŹō£¬ŌŚæÕĘųÖŠŅ׊Ī³ÉAl2O3±”Ĥ£¬NH4Cl·Ö½ā²śÉśµÄHClÄܹ»ĘĘ»µAl±ķĆęµÄAl2O3±”Ĥ£¬ĖłŅŌŌŚAlµÄ·ŪÄ©ÖŠĢķ¼ÓÉŁĮæNH4Cl¹ĢĢå²¢³ä·Ö»ģŗĻ£¬ÓŠĄūÓŚAlNµÄÖʱø”£


 ĘߊĒĶ¼ŹéæŚĖćĖŁĖćĢģĢģĮ·ĻµĮŠ“š°ø
ĘߊĒĶ¼ŹéæŚĖćĖŁĖćĢģĢģĮ·ĻµĮŠ“š°ø ³õ֊ѧŅµæ¼ŹŌµ¼ÓėĮ·ĻµĮŠ“š°ø
³õ֊ѧŅµæ¼ŹŌµ¼ÓėĮ·ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æŌŚĀĮÓėĻ”ĮņĖįµÄ·“Ó¦ÖŠ£¬ŅŃÖŖ10sÄ©ĮņĖįµÄÅØ¶Č¼õÉŁĮĖ0.6 mol/L£¬²»æ¼ĀĒ·“Ó¦¹ż³ĢÖŠČÜŅŗĢå»żµÄ±ä»Æ£¬Ōņ10sÄŚÉś³ÉĮņĖįĀĮµÄĘ½¾ł·“Ó¦ĖŁĀŹŹĒ( )
A. 0.02 mol/(Lmin)B. 1.2 mol/(Lmin)
C. 1.8mol/(Lmin)D. 0.18 mol/(Lmin)
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æ»ÆŗĻĪļXŹĒŅ»ÖÖĻćĮĻ,æɲÉÓĆŅŅĻ©Óė¼×±½ĪŖÖ÷ŅŖŌĮĻ,°“ĻĀĮŠĀ·ĻßŗĻ³É:

ŅŃÖŖ:RX![]() ROH;RCHO£«CH3COOR”ä
ROH;RCHO£«CH3COOR”ä![]() RCH=CHCOOR”ä
RCH=CHCOORӊ
Ēė»Ų“š:
£Ø1£©B£«D”śFµÄ»Æѧ·½³ĢŹ½_______________________________________”£
£Ø2£©XµÄ½į¹¹¼ņŹ½_____”£
£Ø3£©¶ŌÓŚ»ÆŗĻĪļX,ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ____”£
A£®²»ÄÜ·¢ÉśĖ®½ā·“Ó¦ B£®²»ÓėÅØĻõĖį·¢ÉśČ”“ś·“Ó¦
C£®ÄÜŹ¹Br2/CCl4ČÜŅŗĶŹÉ« D£®ÄÜ·¢ÉśŅų¾µ·“Ó¦
£Ø4£©ĻĀĮŠ»ÆŗĻĪļÖŠŹōÓŚFµÄĶ¬·ÖŅģ¹¹ĢåµÄŹĒ______£ØĖ«Ń”£©
A£®![]() B£®
B£®![]()
C£®CH2CHCHCHCHCHCHCHCOOH D£®![]()
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æijӊ»śĪļµÄ½į¹¹¼ņŹ½ČēĶ¼ĖłŹ¾£¬ ĻĀĮŠĖµ·ØÖŠ²»ÕżČ·µÄŹĒ( )

A. 1moløĆÓŠ»śĪļŗĶ¹żĮæµÄ½šŹōÄĘ·“Ó¦×ī¶ąæÉŅŌÉś³É1.5 molH2
B. øĆÓŠ»śĪļĻūŗÄNa”¢NaOH”¢NaHCO3µÄĪļÖŹµÄĮæÖ®±ČĪŖ3”Ć2”Ć2
C. æÉŅŌÓĆĖįŠŌKMnO4ČÜŅŗ¼ģŃéĘäÖŠµÄĢ¼Ģ¼Ė«¼ü
D. øĆÓŠ»śĪļÄܹ»ŌŚ“߻ƼĮ×÷ÓĆĻĀ·¢Éśõ„»Æ·“Ó¦
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĪŖĮĖ¼ģŃéijFeCl2ČÜŅŗŹĒ·ń±äÖŹ£¬Ó¦Ń”ÓƵďŌ¼ĮŹĒ£Ø £©
A.Ļ”ŃĪĖįB.KSCNČÜŅŗC.Ģś·ŪD.ŹÆČļŹŌŅŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æ£Ø1£©Š“³öCH4ŗĶCl2ŌŚ¹āÕÕµÄĢõ¼žĻĀÉś³ÉCH3ClµÄ»Æѧ·“Ó¦·½³ĢŹ½_________
£Ø2£©Š“³öČēĶ¼ĖłŹ¾Ōµē³ŲµÄµē¼«·½³ĢŹ½£ŗ
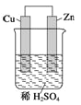
øŗ¼«£ŗ_______________________
Õż¼«£ŗ_______________________
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĻĀĮŠø÷×éĪļÖŹ£ŗ(ÓĆŠņŗÅĢīæÕ)
¢Ł O2ŗĶO3 £» ¢Ś12C Óė14C£» ¢Ū CH3 (CH2) 3 CH3ŗĶ![]()
¢Ü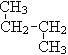 ŗĶ
ŗĶ![]() ;
;
(1)»„ĪŖĶ¬Ī»ĖŲµÄŹĒ_______ £»(2)»„ĪŖĶ¬ĖŲŅģŠĪĢåµÄŹĒ________£»
(3)»„ĪŖĶ¬·ÖŅģ¹¹ĢåµÄŹĒ________£»(4)ŹōÓŚĶ¬Ņ»ĪļÖŹµÄŹĒ________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĻĀĮŠĖµ·ØÕżČ·µÄŹĒ£Ø £©
A. ĶéĢžµÄĶØŹ½ĪŖ CnH2n£«2£¬Ėę n ÖµŌö“ó£¬Ģ¼ŌŖĖŲµÄÖŹĮæ°Ł·Öŗ¬ĮæÖš½„¼õŠ”
B. ŅŅČ²Óė±½µÄŹµŃéŹ½ĪŖ CH£¬ŹĒŗ¬Ģ¼Įæ×īøßµÄĪļÖŹ
C. 1 Ħ¶ū±½Ē”ŗĆÓė 3 Ħ¶ūĒāĘųĶźČ«¼Ó³É£¬ĖµĆ÷Ņ»øö±½·Ö×ÓÖŠÓŠČżøöĢ¼Ģ¼Ė«¼ü
D. n£½7£¬Ö÷Į“ÉĻÓŠ 5 øöĢ¼Ō×ÓµÄĶéĢž¹²ÓŠĪåÖÖ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æijӊ»ś»ÆŗĻĪļAµÄ½į¹¹¼ņŹ½ČēĻĀ£ŗ

£Ø1£©AµÄ·Ö×ÓŹ½ŹĒ________”£
£Ø2£©AŌŚNaOHČÜŅŗÖŠ³ä·Ö¼ÓČČŗó£¬ŌŁ¼ÓČė¹żĮæµÄŃĪĖįĖį»Æŗó£¬æɵƵ½B”¢C”¢DŗĶ![]() ĖÄÖÖÓŠ»śĪļ£¬ĒŅĻą¶Ō·Ö×ÓÖŹĮæ“󊔹ŲĻµĪŖB>C>D£¬ŌņB”¢C”¢DµÄ½į¹¹¼ņŹ½·Ö±šŹĒB________£»C________£»D________”£
ĖÄÖÖÓŠ»śĪļ£¬ĒŅĻą¶Ō·Ö×ÓÖŹĮæ“󊔹ŲĻµĪŖB>C>D£¬ŌņB”¢C”¢DµÄ½į¹¹¼ņŹ½·Ö±šŹĒB________£»C________£»D________”£
£Ø3£©ŌŚBµÄĶ¬·ÖŅģ¹¹ĢåÖŠ£¬ŹōÓŚ1,3,5ȿȔ“ś±½µÄ»ÆŗĻĪļµÄ½į¹¹¼ņŹ½__________________”£
£Ø4£©Š“³öBÓėĢ¼ĖįĒāÄĘ·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ________________________________________”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com