
��ˮ�Ǿ����Դ���⣬��ˮ���������ۺ����þ�����Ҫ���塣

���������գ�
��1���ȼҵ��Ҫ��ʳ��Ϊԭ�ϡ�Ϊ�˳�ȥ�����е�Ca2+��Mg2+��SO42������ɳ���ɽ���������ˮ��Ȼ��������в�������ȷ�IJ���˳���� ��
�ٹ��� �ڼӹ�����NaOH��Һ �ۼ����������� �ܼӹ�����Na2CO3��Һ
�ݼӹ�����BaCl2��Һ
a���ڢݢܢ٢� b���٢ܢڢݢ� c���ܢڢݢۢ� d���ݢڢܢ٢�
��2����ʵ�����п�������ȡ�ķ�����ȡ�壬��ѡ�õ��Լ���________________��������Ҫ������������____________________��
��3����������������ữ�����Cl2�����ʵ�ԭ���� ��
��4������II��Ӧ�����ӷ���ʽ__________________________________________��
��5��Mg(OH)2�����л���Ca(OH)2����ѡ��__________��Һ����ϴ�ӳ�ȥ��
 �Ͻ�ƽ���Ȿϵ�д�
�Ͻ�ƽ���Ȿϵ�д� ����ѧ��Ӧ�����ϵ�д�
����ѧ��Ӧ�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����9�֣�������������Ҫ�Ĺ�ҵԭ�Ϻ��ܼ���ͨ�����������Ҵ������ᡣijѧϰС������ͼ1װ����ȡ���������ֲ�Ʒ���ٷ������������ĺ�����
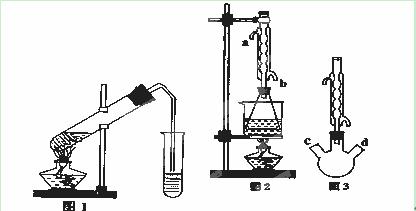
| �������� | �Ҵ� | ���� | |
| �е� | 77.1�� | 78.5�� | 117.9�� |
�����������£�
��I��ȷ����20.0g����������Ʒ����ƿ�У���0.50mol��L��1NaOH�ζ�����̪��ָʾ�������յ�ʱ����NaOH��Һ�����Ϊ40.0mL
��II����ȡ20.0g���������ֲ�Ʒ��250mL��ƿ�У�����100mL 2.1mol��L��1NaOH��Һ��Ͼ��Ⱥ�װ�������䣬��ˮԡ�ϼ��Ȼ���Լ1Сʱ��װ����ͼ2��ʾ������ȴ����0.50mol��L��1HCl�ζ�������NaOH���յ�ʱ������������Ϊ20.0mL��
�ش��������⣺
��1��ʵ�飨II������ˮ����ˮ���ܵ� ����a��b���ܿ�ͨ�롣
��2������ʵ�飨I������II�����������ݼ���ֲ�����������������������Ϊ ��
��3��ʵ�������ͬѧ�ǶԴֲ�Ʒ�����������ĺ������߽������ۡ�
��������Ϊ��ʵ�飨II�������������齫ͼ2�е���ƿ��Ϊ����ƿ��װ����ͼ3��������ƿ��c��d��װ����ص�����������ǡ���IJ�����������߲ⶨ�ľ�ȷ�ȡ�����Ϊ������ƿ��c��d��װ����ص�����������ǣ� ������ĸ����
A��װ���¶ȼƣ��ϸ���Ʒ�Ӧ�¶�
B��ʵ���о�����ƿ�ڣ��ò������н���
C���ڷ�Ӧ���ڣ������Ȱ�װ�ķ�Һ©������һ������NaOH��Һ
�ڻ���ͬѧ��Ϊ�Ľ�������������ȡװ�ã�ͼ1��������߲��ʡ�������һ���Ľ�����
����6�֣���ͼ������50 mL����KMnO4����Һ�Ĺ���ʾ��ͼ��
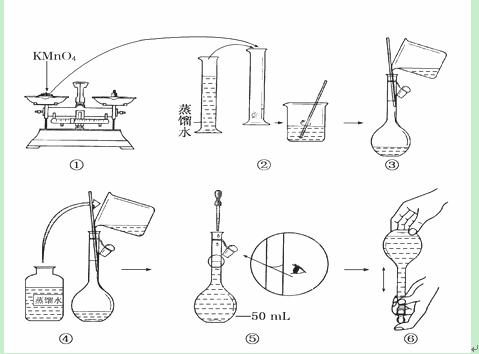
(1)����۲�ͼʾ�ж����в���ȷ�IJ�����________(�����)��
(2)����ȷ��50 mL��Һ�����������____________________________________(������)��
(3)�������ͼʾ�IJ��������Ƶ���Һ����ʵ�飬��������������ȷ������£�����õ�ʵ������________(�ƫ��ƫС��)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪������������ԣ�HA>HB���ڳ��������и���Ƚ�����ȷ����
A�������ʵ���Ũ�ȵ�HA��Һ��NaB��Һ������Ļ��Һ�У�
2c(Na+) =c(A-)+c(B-)+c(HA)+c(HB)
B��pH��ȵ�NaA��KB����Һ�У�[c(Na+)-c(A-)] < [c(K+)-c(B-)]
C��pH=9��������Һ ��NaB ��NH3��H2O ��NaOH����ˮ�������c(OH-)����>��>��
D. ������Һ�� 0.1mol/L HA��Һ ��0.1mol/L HB��Һ����ˮϡ����pH ��ͬ,���ˮ����ǰ��С�ں���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����ӵ�����ԼΪ6.02��1023mol��1������˵������ȷ����
A��1.0L 1.0mol·L��1CH3COOH��Һ�У�CH3COOH������Ϊ6.02��1023
B�� Na��H2O��Ӧ����1.12L H2(��״��),��Ӧ��ת�Ƶĵ�����Ϊ6.02��1023
C��32 g S8�����к��е�S—S������Ϊ6.02��1023 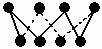
D��22.4 L N2�������ķ�����Ϊ6.02��1023
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���и���Һ�У������ӿ��ܴ����������
A�����д���Ba2������Һ�У�HCO3����Fe3����Ag����SO42����SCN��
B���μ���ɫ��̪��Һ��Ϊ��ɫ����Һ�У�Na����CO32����K����ClO����AlO2��
C����ˮ�����c(OH��)=10��14mol·L��1 ����Һ�У�CH3COO����C6H5O����Na����K��
D���������ۺ������������Һ�У�NH4����Na����NO3����Cl����HS��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijԪ�ص�ԭ��������Ӳ��Ų���5s25p1,��Ԫ�ػ��仯���ﲻ���ܾ��е������� �� ��
A����Ԫ�ص����ǵ��� B����Ԫ�ص�����һ���������������ᷴӦ
C����Ԫ�ص��������ˮ�����Լ��� D����Ԫ�ص�����ϼ۳�+5�� �� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������������Ȼ�ѧ����ʽ�ֱ�Ϊ�� �� ��
2H2(g)+O2(g)==2H2O(l)����H= ��571.6kJ��mol-1
CH4(g)+2O2(g)==CO2(g)+2H2O(l)����H= ��89.3kJ��mol-1
�����������鰴һ��������ϣ����������ܶ�����ͬ�����µ����ܶȵ�һ�룬��9.8g�û��������ȫȼ�շų�������Ϊ
A��28.58kJ B��82.16 kJ C��53. 58 kJ D��89.3 kJ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪X��Y��ZΪ����ԭ������������Ԫ�أ�����������Ӧˮ������������ǿ���ǣ�HXO4��H2YO4��H3ZO4��������˵������ȷ����
A����̬�⻯����ȶ��ԣ�HX��H2Y��ZH3 B���ǽ��������ԣ�Z��Y��X
C�����ʵ������ԣ�X2��Y��Z D��ԭ��������Ӳ��ϵĵ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���º��ݵ�������,������Ϊ���淴ӦI2(g)+H2(g) 2HI(g)�ﵽƽ��״̬�ı�־����(����)
2HI(g)�ﵽƽ��״̬�ı�־����(����)
A.1 mol H—H������,ͬʱ��2 mol H—I������ B.�������������ѹǿ���ٱ仯
C.H2�����ʵ���Ũ�Ȳ��ٱ仯 D.����������ɫ���ٱ仯
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com