
ДПМю(Na2CO3)дкЩњВњЩњЛюжаОпгаЙуЗКЕФгУЭОЁЃвдЯТЪЧЪЕбщЪвФЃФтжЦМюдРэжЦШЁNa2CO3ЕФСїГЬЭМ

вбжЊ:ЯђБЅКЭЪГбЮЫЎжаЭЈШыNH3ЃЌCO2КѓЗЂЩњКЭЗДгІЮЊNaClЃЋNH3ЃЋCO2ЃЋH2O NaHCO3Ё§ЃЋNH4Cl,ЧыЛиД№вдЯТЮЪЬтЃК
NaHCO3Ё§ЃЋNH4Cl,ЧыЛиД№вдЯТЮЪЬтЃК
ЃЈ1ЃЉДжбЮжаКЌгаЕФдгжЪРызггаCa2+ЃЌMg2+ЃЌSO42-ЕШЁЃ
ОЋжЦГ§дгЕФВНжшЫГађaЁњ_______Ёњ________Ёњ________Ёњb(ЬюзжФИБрКХЃЉЁЃ
aЃЎДжбЮШмНтЃЌТЫШЅГСдќЃЎbЃЎМгШыбЮЫсЕїpHЃЛcЃЎМгШыBa(OH)2ШмвКЃЛdЃЎМгШыNa2CO3ШмвКЃЛeЃЎЙ§ТЫ
ЯђБЅКЭЪГбЮЫЎжаЯШЭЈШыNH3ЃЌКѓЭЈШЫCO2ЃЌРэгЩЪЧ_____________________ЁЃ
ЃЈ2ЃЉзЦЩеЙЬЬхAжЦNa2CO3дк_____ЬюзжФИађКХЃЉжаНјааЁЃ
aЃЎлслі bЃЎеєЗЂУѓ cЃЎЩеБ dЃЎзЖаЮЦП
жЄУїТЫвКAжаКЌгаNH4+ЕФЗНЗЈЪЧ__________________________________________________________ЁЃ
ЖдТЫвКAНјаажиНсОЇФмЙЛЛёЕУNH4HCO3ЃЌЯђpH=13КЌNa+ЃЌK+ЕФШмвКжаМгШыЩйСПNH4HCO3ЁЃЪЙpHНЕЕЭЃЌЗДгІЕФРызгЗНГЬЪН____________________________________ЁЃ
ЃЈ3ЃЉЯТЭМзАжУжаГЃгУгкЪЕбщЪвжЦБИCO2ЕФЪЧ_____(ЬюзжФИБрКХЃЉЃЛгУbЪОвтЕФзАжУжЦБИNH3ЃЌЗжвКТЉЖЗжаЪЂЗХЕФЪдМС______(ЬюЪдМСУћГЦЃЉЃЌЩеЦПФкПЩМгШыЕФЙЬЬхЪдМС__________ЃЈЬюЪдМСУћГЦЃЉЁЃ

ЃЈ4ЃЉвЛжжЬьШЛМюОЇЬхГЩЗжЪЧaNa2CO3ЁЄbNa2CO3ЁЄcH2OЃЌФГЭЌбЇРћгУЯТСаЬсЙЉЕФЪдМСЃЌЩшМЦСЫШчЯТМђЕЅКЯРэВтЖЈNa2CO3ЕФжЪСПЗжЪ§ЕФЪЕПЅЗНАИЁЃЃЈвЧЦїздбЁЃЉЧыАбЪЕбщЗНАИЬюШЋЃКЙЉбЁдёЕФЪдМСЃК1.0mol/LH2SO4ШмвКЁЂ1.0mol/L BaCl2ШмвКЁЂЯЁАБЫЎЁЂМюЪЏЛвЁЂCa(OH)2ШмвКЁЂеєСѓЫЎ
ЂйГЦШЁm1gвЛЖЈСПЬьШЛМюОЇЬхбљЦЗЃЌШмгкЪЪСПеєСѓЫЎжаЁЃ
Ђк_________________________________________________________________ЁЃ
Ђл_________________________________________________________________ЁЃ
ЂмМЦЫуЬьШЛМюОЇЬхжаКЌNa2CO3ЕФжЪСПЗжЪ§ЁЃ
ЃЈЙВ16ЗжЃЉЃЈ1ЃЉc d eЃЈ2ЗжЃЉЃЛNH3взШмгкЫЎЃЌгаРћгкЮќЪеШмНтЖШВЛДѓЕФCO2ЃЈ2ЗжЃЉ
ЃЈ2ЃЉ a ЃЈ1ЗжЃЉЃЛШЁЩйСПТЫвКAгкЪдЙмжаЃЌМгШызуСПNaOHШмвКВЂМгШШЃЌВњЩњФмЪЙЪЊШѓЕФКьЩЋЪЏШяЪджНБфРЖЩЋЕФЦјЬхЃЌжЄУїAжаКЌгаNH4+ЃЈ2ЗжЃЉЃЛNH4++HCO3-+2OH-ЃНNH3ЁЄH2O+CO32-+H2O ЃЈ2ЗжЃЉ
ЃЈ3ЃЉb c ЃЈ2ЗжЃЉХЈАБЫЎ( 1Зж)ЁЂЩњЪЏЛвЃЈЛђNaOHЙЬЬхЛђМюЪЏЛвЃЉ( 1Зж)
ЃЈ4ЃЉ ЂкМгШызуСПЯЁСђЫсВЂЮЂШШЃЌВњЩњЕФЦјЬхЭЈЙ§зуСПЕФГЮЧхЪЏЛвЫЎЃЌЃЈ1ЗжЃЉ
ЂлЙ§ТЫЁЂЯДЕгЁЂИЩдяЁЂГЦСПГСЕэЃЛЃЈ2ЗжЃЉ
ЁОНтЮіЁП
ЪдЬтЗжЮіЃКЃЈ1ЃЉCa2ЃЋгУЬМЫсФЦГ§ШЅЃЌMg2ЃЋгУOHЃГ§ШЅЃЌSO42ЃгУBa2ЃЋГ§ШЅЃЌзюКѓМгШыбЮЫсЫсЛЏЁЃЕЋгЩгкЙ§СПЕФBa2ЃЋвЊгУЬМЫсФЦРДГ§ЃЌЫљвдЬМЫсФЦБиашЗХдкзюКѓМгШыЃЌЫљвде§ШЗЕФВйзїЫГађЪЧacdebЃЛгЩгкNH3взШмгкЫЎЃЌCO2дкЫЎжаЕФШмНтЖШВЛДѓЃЌЫљвдЯШЭЈШыАБЦјгаРћгкЮќЪеШмНтЖШВЛДѓЕФCO2ЁЃ
ЃЈ2ЃЉЙЬЬхзЦЩедклсліжаНјааЃЌвђДЫД№АИбЁaЃЛгЩгкяЇбЮФмКЭЧПМюЗДгІЩњГЩАБЦјЃЌЫљвдПЩвдЭЈЙ§МьбщАБЦјРДМьбщNH4ЃЋ,МДе§ШЗЕФВйзїЪЧШЁЩйСПТЫвКAгкЪдЙмжаЃЌМгШызуСПNaOHШмвКВЂМгШШЃЌВњЩњФмЪЙЪЊШѓЕФКьЩЋЪЏШяЪджНБфРЖЩЋЕФЦјЬхЃЌжЄУїAжаКЌгаNH4+ЃЛгЩгкNH4+ЁЂHCO3-КЭOHЃОљЗДгІЃЌДгЖјЪЙШмвКЕФpHНЕЕЭЃЌЗДгІЕФРызгЗНГЬЪНЪЧNH4++HCO3-+2OH-ЃНNH3ЁЄH2O+CO32-+H2OЁЃ
ЃЈ3ЃЉЪЕбщЪвжЦШЁCO2РћгУЕФЪЧЪЏЛвЪЏКЭбЮЫсЗДгІЃЌЧвЗДгІВЛашвЊМгШШЃЌЫљвдзАжУжаЪЪКЯжЦШЁCO2ЕФЪЧбЁЯюbcЃЛдкАБЫЎжаДцдкЦНКтNH3ЃЋH2O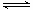 NH3ЁЄH2O
NH3ЁЄH2O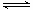 NH4ЃЋЃЋOHЃЃЌЫљвдПЩвдЯђХЈАБЫЎжаМгШыЩњЪЏЛвЃЈЛђNaOHЙЬЬхЛђМюЪЏЛвЃЉРДжЦБИАБЦјЃЌМДЗжвКТЉЖЗжаЪЂЗХЕФЪЧХЈАБЫЎЃЌЩеЦПФкПЩМгШыЕФЙЬЬхЪдМСЩњЪЏЛвЃЈЛђNaOHЙЬЬхЛђМюЪЏЛвЃЉЁЃ
NH4ЃЋЃЋOHЃЃЌЫљвдПЩвдЯђХЈАБЫЎжаМгШыЩњЪЏЛвЃЈЛђNaOHЙЬЬхЛђМюЪЏЛвЃЉРДжЦБИАБЦјЃЌМДЗжвКТЉЖЗжаЪЂЗХЕФЪЧХЈАБЫЎЃЌЩеЦПФкПЩМгШыЕФЙЬЬхЪдМСЩњЪЏЛвЃЈЛђNaOHЙЬЬхЛђМюЪЏЛвЃЉЁЃ
ЃЈ4ЃЉгЩгкЬМЫсФЦФмКЭЯЁСђЫсЗДгІЩњГЩCO2ЦјЬхЃЌЖјCO2ФмКЭГЮЧхЕФЪЏЛвЫЎЗДгІЩњГЩЬМЫсИЦГСЕэЃЌвђДЫПЩвдЭЈЙ§ГЦСПЬМЫсИЦЕФжЪСПРДМЦЫуЬМЫсФЦЕФжЪСПЃЌМДе§ШЗЕФВйзїЪЧЂкМгШызуСПЯЁСђЫсВЂЮЂШШЃЌВњЩњЕФЦјЬхЭЈЙ§зуСПЕФГЮЧхЪЏЛвЫЎЃЛЂлЙ§ТЫЁЂЯДЕгЁЂИЩдяЁЂГЦСПГСЕэЃЛ
ПМЕуЃКПМВщДжбЮЕФЬсДПЃЛЬМЫсФЦЕФжЦБИЃЛвЧЦїбЁдёЃЛCO2КЭАБЦјЕФжЦБИЃЛNH4+МьбщвдМАЮяжЪКЌСПВтЖЈЕФЪЕбщЗНАИЩшМЦЕШ



| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃКдФЖСРэНт
| ЮТЖШШмНтЖШбЮ | 0Ёц | 10Ёц | 20Ёц | 30Ёц | 40Ёц | 50Ёц | 60Ёц | 100Ёц |
| NaCl | 35.7 | 35.8 | 36.0 | 36.3 | 36.6 | 37.0 | 37.3 | 39.8 |
| NH4HCO3 | 11.9 | 15.8 | 21.0 | 27.0 | -Ђй | - | - | - |
| NaHCO3 | 6.9 | 8.1 | 9.6 | 11.1 | 12.7 | 14.5 | 16.4 | - |
| NH4Cl | 29.4 | 33.3 | 37.2 | 41.4 | 45.8 | 50.4 | 55.3 | 77.3 |
| c(V2-V1)M |
| 1000W |
| c(V2-V1)M |
| 1000W |
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃКдФЖСРэНт
ЫФжжбЮдкВЛЭЌЮТЖШЯТЕФШмНтЖШЃЈg/100 gЫЎЃЉБэ
ЮТЖШ ШмНтЖШ бЮ | 0 Ёц | 10 Ёц | 20 Ёц | 30 Ёц | 40 Ёц | 50 Ёц | 60 Ёц | 100 Ёц |
NaCl | 35.7 | 35.8 | 36.0 | 36.3 | 36.6 | 37.0 | 37.3 | 39.8 |
NH4HCO3 | 11.9 | 15.8 | 21.0 | 27.0 | ЁЊЂй | ЁЊ | ЁЊ | ЁЊ |
NaHCO3 | 6.9 | 8.1 | 9.6 | 11.1 | 12.7 | 14.5 | 16.4 | ЁЊ |
NH4C | l29.4 | 33.3 | 37.2 | 41.4 | 45.8 | 50.4 | 55.3 | 77.3 |
ЂйЃО35Ёц NH4HCO3ЛсгаЗжНт
ЧыЛиД№ЃК
ЃЈ1ЃЉЗДгІЮТЖШПижЦдк30ЁЊ35 ЁцЃЌЪЧвђЮЊШєИпгк35 ЁцЃЌдђ__________________________ЃЌШєЕЭгк30 ЁцЃЌдђ______________________________________ЃЛЮЊПижЦДЫЮТЖШЗЖЮЇЃЌВЩШЁЕФМгШШЗНЗЈЮЊ__________________________________________________________ЁЃ
ЃЈ2ЃЉМгСЯЭъБЯКѓЃЌМЬајБЃЮТ30ЗжжгЃЌФПЕФЪЧ______________________________________ЁЃОВжУКѓжЛЮіГіNaHCO3ОЇЬхЕФдвђЪЧ___________________________________________ЁЃгУеєСѓЫЎЯДЕгNaHCO3ОЇЬхЕФФПЕФЪЧГ§ШЅ_______________________________________дгжЪЃЈвдЛЏбЇЪНБэЪОЃЉЁЃ
ЃЈ3ЃЉЙ§ТЫЫљЕУЕФФИвКжаКЌга______________________________________ЃЈвдЛЏбЇЪНБэЪОЃЉЃЌашМгШы___________________ЃЌВЂзїНјвЛВНДІРэЃЌЪЙNaClШмвКбЛЗЪЙгУЃЌЭЌЪБПЩЛиЪеNH4ClЁЃ
ЃЈ4ЃЉВтЪдДПМюВњЦЗжаNaHCO3КЌСПЕФЗНЗЈЪЧЃКзМШЗГЦШЁДПМюбљЦЗW gЃЌЗХШызЖаЮЦПжаМгеєСѓЫЎШмНтЃЌМг1ЁЊ2ЕЮЗгЬЊжИЪОМСЃЌгУЮяжЪЕФСПХЈЖШЮЊc( molЁЄL-1)ЕФHClШмвКЕЮЖЈжСШмвКгЩКьЩЋЕНЮоЩЋЃЈжИЪО![]() +H+====
+H+====![]() ЗДгІЕФжеЕуЃЉЃЌЫљгУHClШмвКЬхЛ§ЮЊV1 mLЃЌдйМг1ЁЊ2ЕЮМзЛљГШжИЪОМСЃЌМЬајгУHClШмвКЕЮЖЈжСШмвКгЩЛЦБфГШЃЌЫљгУHClШмвКзмЬхЛ§ЮЊV2 mLЁЃаДГіДПМюбљЦЗжаNaHCO3жЪСПЗжЪ§ЕФМЦЫуЪНЃК
ЗДгІЕФжеЕуЃЉЃЌЫљгУHClШмвКЬхЛ§ЮЊV1 mLЃЌдйМг1ЁЊ2ЕЮМзЛљГШжИЪОМСЃЌМЬајгУHClШмвКЕЮЖЈжСШмвКгЩЛЦБфГШЃЌЫљгУHClШмвКзмЬхЛ§ЮЊV2 mLЁЃаДГіДПМюбљЦЗжаNaHCO3жЪСПЗжЪ§ЕФМЦЫуЪНЃК
w(NaHCO3)=___________________ЁЃ
?
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2013-2014бЇФъЙуЖЋЪЁИпШ§ЩЯбЇЦкЦкжаПМЪдЛЏбЇЪдОэЃЈНтЮіАцЃЉ ЬтаЭЃКбЁдёЬт
50ЁцЪБЃЌЯТСаИїШмвКжаЃЌРызгЕФЮяжЪЕФСПХЈЖШЙиЯЕе§ШЗЕФЪЧ
AЃЎБЅКЭДПМю(Na2CO3)ШмвКжаЃКc(Na+)ЃН2c(CO32Ѓ)
BЃЎpHЃН4ЕФДзЫсжаЃКc(H+)ЃН1.0ЁС10Ѓ4molЁЄLЃ1
CЃЎ0.10mol/LДзЫсФЦШмвКжаЃКc(Na+)ЃЋc(H+)ЃНc(CH3COOЃ)ЃЋc(OHЃ)
DЃЎpHЃН12ЕФДПМюШмвКжаЃКc(OHЃ)ЃН1.0ЁС10Ѓ2molЁЄLЃ1
ВщПДД№АИКЭНтЮі>>
АйЖШжТаХ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com