
| 1 |
| 2 |
| 1 |
| 2 |
| 1 |
| 2 |
| 1 |
| 2 |
| 1 |
| 2 |
| 1 |
| 2 |
| 1 |
| 2 |


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| 1 |
| 2 |
| 1 |
| 2 |
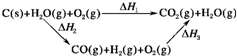
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
C(s)+O2(g)====CO2(g) ��H=-393.5 kJ��mol-1 ��1��
H2(g)+![]() O2(g)====H2O(g) ��H=-242.0 kJ��mol-1 ��2��
O2(g)====H2O(g) ��H=-242.0 kJ��mol-1 ��2��
CO(g)+12O2(g)====CO2(g) ��H=-283.0 kJ��mol-1 ��3��
��ش�
(1)�����������ݣ�д��C(s)��ˮ������Ӧ���Ȼ�ѧ����ʽ��___________________��
(2)�ȽϷ�Ӧ�����ݿ�֪��1 mol CO(g)��1 mol H2(g)��ȫȼ�շų�������֮�ͱ�1 mol C(s)��ȫȼ�շų��������ࡣ��ͬѧ�ݴ���Ϊ��úת��Ϊˮú������ʹúȼ�շų����������������ͬѧ���ݸ�˹������������ѭ��ͼ��
���ݴ���Ϊ��úת��Ϊˮú����ȼ�շų���������úֱ��ȼ�շų���������ȡ���
��������ס�����ͬѧ�۵���ȷ����____________(��ס����ҡ�)���жϵ�������__________��
(3)��úת��Ϊˮú����Ϊȼ�Ϻ�úֱ��ȼ������кܶ��ŵ㣬���о����е������ŵ�______________��
(4)ˮú������������������ȼ�ϣ�Ҳ����Ҫ���л�����ԭ�ϡ�CO��H2��һ�������¿��Ժϳɣ��ټ״� �ڼ�ȩ �ۼ��� �����ᡣ�Է�����CO��H2��1��1������Ȼ�Ϸ�Ӧ���ϳ�����______________(�����)����ʱ���������㡰��ɫ��ѧ����Ҫ����ȫ����ԭ���е�ԭ�ӣ�ʵ�����ŷš�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ͬ���� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
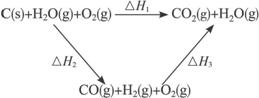
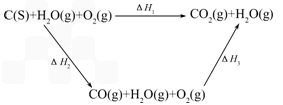
��úת��Ϊˮú������Ҫ��ѧ��ӦΪC(s)+H2O(g)![]() CO(g)+H2(g)��C(s)��CO(g)��H2(g)��ȫȼ�յ��Ȼ�ѧ����ʽΪ��
CO(g)+H2(g)��C(s)��CO(g)��H2(g)��ȫȼ�յ��Ȼ�ѧ����ʽΪ��
C(s)+O2(g)=CO2(g)������ ��H=-393��5kJ?mol-1
H2(g)+![]() O2(g)=H2O(g)���� ��H=-242.0kJ?mol-1
O2(g)=H2O(g)���� ��H=-242.0kJ?mol-1
CO(g)+![]() O2(g)=CO2(g)���� ��H=-283.0kJ?mol-1
O2(g)=CO2(g)���� ��H=-283.0kJ?mol-1
��ش�
(1)�����������ݣ�д��C(s)��ˮ������Ӧ���Ȼ�ѧ����ʽ��
�� ������������������������������������������������������������������������
(2)�ȽϷ�Ӧ�����ݿ�֪��1molCO(g)��1molH2(g)��ȫȼ�շų�������֮�ͱ�1molC(s)��ȫȼ�շų��������ࡣ��ͬѧ�ݴ���Ϊ��úת��Ϊˮú������ʹúȼ�շų����������������ͬѧ���ݸ�˹������������ѭ��ͼ��

�� ���ݴ���Ϊ��úת��Ϊˮú����ȼ�շų���������úֱ��ȼ�շų���������ȡ���
��������ס�����ͬѧ�۵���ȷ���� ������(��ס����ҡ�)���жϵ�������������������
����������������������������������������������������������������������������������
(3)��úת��Ϊˮú����Ϊȼ�Ϻ�úֱ��ȼ������кܶ��ŵ㣬���о����е������ŵ���������
������������������������������������ ����������������������������������������������
(4)ˮú������������������ȼ�ϣ�Ҳ����Ҫ���л�����ԭ�ϡ�CO��H2��һ�������¿��Ժϳɣ��ټ״� �ڼ�ȩ �ۼ��� �����ᡣ�Է�����CO��H2��1��1������Ȼ�Ϸ�Ӧ���ϳ��������� (�����)����ʱ���������㡰��ɫ��ѧ����Ҫ����ȫ����ԭ���е�ԭ�ӣ�ʵ�����ŷš�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�찲��ʡʡ����У�����ڶ���������ѧ�Ծ� ���ͣ������
(14�֣�������������������������쳣����Ƶ�����磺���ϳ��������µĸɺ�������Ƶ����ɳ�����ȣ�ʹ���������ٴγ�Ϊ���ڵĽ��� �ǶԻ���Ӱ��ϴ�Ķ������壬�����ǵĺ������ƺ��������Ż��������滷������Ч;����
�ǶԻ���Ӱ��ϴ�Ķ������壬�����ǵĺ������ƺ��������Ż��������滷������Ч;����
(1�����������У������ڽ��ʹ����е� Ũ�ȵ���__________ (ѡ����ĸ)��
Ũ�ȵ���__________ (ѡ����ĸ)��
a. ֱ����ú��ȼ��
b. ��˽�ҳ�
c. ���������������̻����
d. �о����Է�̫����ʹ֮�߽�Ѱ�����ռ�
(2 )��CO��H2��ԭ�Ͽ��Ժϳɼ״�����ΪҺ��ȼ�ϡ���֪��

��д���úϳ�����CO��H2)�ϳ�1molҺ̬�״����Ȼ�ѧ��Ӧ����ʽ��__________
(3) ���õ绯ѧԭ����CO��SO2R��Ϊ��Ҫ����ԭ�ϣ�װ����ͼ��ʾ��
����AΪCO,BΪH2, CΪCH3OH,��ͨ��CO��һ��Ϊ_____��
����AΪSO2,BΪO2,CΪH2SO4,���ĵ缫��ӦʽΪ��__________
(4) ����֪���ܱ������У� �����£�
�����£�
 ,��ƽ�ⳣ��K=13.3��
,��ƽ�ⳣ��K=13.3��
���˷�Ӧ�ﵽƽ��ʱ����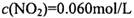 ����
����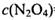 =_______________(������λ��Ч����)��
=_______________(������λ��Ч����)��
�����ı�������ϵ��ij���������ﵽ�µ�ƽ���û�������� ��
�� ����ı��������____________________
����ı��������____________________
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com