
����Ŀ�������������У�����кͼ���Һ������о�����ʮ����Ҫ�����塣
��1�������£���2��һԪ��ֱ��NaOH��Һ�������ϣ�ʵ���������£�
��� | c��һԪ�ᣩ | c��NaOH�� | �����Һ��pH |
�� | c(HY)=0.1mol/L | 0.1mol/L | pH=7 |
�� | c(HZ)=0.1mol/L | 0.1mol/L | pH=9 |
������ʵ����HYΪ_________����ǿ�������ᣬ0.1 moL/L HY��Һ��pH=_____________��
������ʵ���з�����Ӧ�����ӷ���ʽΪ_________________________________��
������ʵ��������Һ����ˮ�������c(OH-)=________ mol/L��
��2�������£���0.1000mol/L NaOH��Һ�ֱ�ζ�20.00mL0.1000mol/LHCl��Һ��20.00mL 0.1000mol/LCH3COOH��Һ���õ�2���ζ����ߣ�����ͼ��ʾ��

���ζ�HCl��Һ��������___________������I����������
��a=__________ mL��
��c(Na+)=c(CH3COO-)�ĵ���_____________��
��E���Ӧ����Ũ���ɴ�С��˳��Ϊ_________________��
��3����һ����ijŨ�ȵ�NaOH��Һ������MgCO3����Һ�У��������ɸ����ܵ�Mg(OH)2���÷�Ӧ�����ӷ���ʽΪ_____________________________��
���𰸡� ǿ 1 HZ + OH-== H2O + Z- 10-5 �� 20.00 D c(Na+)> c(CH3COO-) > c(OH��) > c(H+) MgCO3 +2OH-==Mg(OH)2 ��+ CO32-
����������1���ٸ��ݱ��������ж�HZΪ������ʣ���Һ�в��ֵ��룬HYΪǿ�ᣬ��ȫ���룻
�ڵ��������Ũ�ȵ�����������HZ��Ӧ����ǿ��������NaZ���ݴ���д����ʽ��
�۵��������Ũ�ȵ�����������HZ��Ӧ����ǿ��������NaZ��Z-���Ӳ���ˮ�⣬��Һ��ʾ���ԣ�NaZ��Һ�е�����������Ϊˮ����ģ�
��2���ٴ���Ϊ������ʣ�����Һ�в��ֵ���������ӣ����Եζ�ǰ������Һ��pH��1��HCl��ǿ����ʣ���ȫ���룬pH��1��
��B��ʱ��Һ��ʾ���ԣ��������������ǡ�÷�Ӧ��
�۸��ݵ���غ������
�ܸ��ݵ���غ������
��3���������ƺ�̼��þ��Ӧ����������þ��̼���ƣ�
��1���ɱ������ݿ�֪�����������Ũ�ȵ�HZ��NaOH��Ӧʱ������ǡ�÷�Ӧ����NaZ����Һ��pH=9��˵��NaZΪǿ�������Σ�HZΪ������ʣ�����Һ�в��ֵ��룻���������Ũ�ȵ�HY��NaOH��Ӧʱ������ǡ�÷�Ӧ����NaY����Һ��pH=7��˵��NaYΪǿ��ǿ���Σ�HYΪ������ʣ���
�ټ���ʵ����HYΪǿ�ᣬ0.1 moL/LHY��Һ��������Ũ����0.1mol/L������Һ��pH=1��
��HZ�����ᣬ������ʵ���з�����Ӧ�����ӷ���ʽΪHZ+OH-=H2O+Z-��
��NaZΪǿ�������Σ�Z-���Ӳ���ˮ�⣺Z-+H2O![]() HZ+OH-����Һ��ʾ���ԣ�NaZ��Һ�е�����������Ϊˮ����ģ�����ˮ���������������Ũ��Ϊ��c��OH-��=1��10-5mol/L��
HZ+OH-����Һ��ʾ���ԣ�NaZ��Һ�е�����������Ϊˮ����ģ�����ˮ���������������Ũ��Ϊ��c��OH-��=1��10-5mol/L��
��2���ٴ���Ϊ���ᣬ����Һ�в��ֵ���������ӣ�����0.1000mol/L CH3COOH��Һ��������Ũ��С��0.1000mol/L����Һ��pH����1������ͼ���ʾ�ζ�������Һ�ĵζ����ߣ�ͼ���ʾ�ζ�HCl��Һ�����ߣ�
�ڸ���ͼ���֪B��ʱ��Һ�����ԣ��������������ǡ�÷�Ӧ����a=20.00 mL��
�۸��ݵ���غ��֪��Һ�д���c(Na+)+c(H+)=c(CH3COO-)+c(OH��)��c(Na+)=c(CH3COO-)������Һ��c(H+)=c(OH��)������Һ�����ԣ���ѡD��
��E���������������ǡ�÷�Ӧ���ɴ����ƣ����������ˮ�⣬��Һ�Լ��ԣ����Ӧ����Ũ���ɴ�С��˳��Ϊc(Na+)��c(CH3COO-)��c(OH��)��c(H+)��
��3����һ����ijŨ�ȵ�NaOH��Һ������MgCO3����Һ�У��������ɸ����ܵ�Mg(OH)2����һ�ַ�Ӧ����̼���ƣ��÷�Ӧ�����ӷ���ʽΪMgCO3+2OH-=Mg(OH)2��+CO32-��


 �߲������Ӧ��һ��ͨϵ�д�
�߲������Ӧ��һ��ͨϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ʵ��װ�ý�����Ӧʵ�飬�ܴﵽʵ��Ŀ�ĵ���

A. ��ͼ2��ʾװ����ȡ��������
B. ��ͼ1��ʾװ�ó�ȥCl2�к��е�����HCl
C. ѡ����ʵ��Լ�����ͼ4��ʾװ�ÿɷֱ���ȡ����CO2��NO��O2
D. ��ͼ3��ʾװ����ȡ����������CO2����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ˮ�м�������������(�¶Ȳ���)��������˵����ȷ���ǣ� ��
A.�ٽ���ˮ�ĵ���B.c(H��)��С
C.ˮ��c(H��)��c(OH��)�ij˻�����D.c(OH��)��С
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ͼ�dz��������IJ��ֽṹ��

��1�����������A__________��B__________��C__________��D__________��
��2��ʹ��ǰ�����Ƿ�©ˮ��������____________������ĸ����
������֪������������ͭ��Ksp=2��10-20��
��3��ij����ͭ��Һ��c(Cu2+)=0.02mol/L����Ҫ����Cu(OH)2������Ӧ������ҺpHʹ֮����_________��
��4��Ҫʹ0.2mol/ LCuSO4��Һ��Cu2+������Ϊ��ȫ��Cu2+Ũ�Ƚ���ԭ����ǧ��֮һ������Ӧ����Һ�����NaOH��Һʹ��ҺpHΪ______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����η�ֹ������ʴ�ǹ�ҵ���о����ص����ݡ�Ϊ�о�������ʴ,ijͬѧ����̽��ʵ��,����ͼ��ʾ,�����ڢ٢ڢ����ֲ�ͬ�Ļ�����

��ش�:
��1��������ʴ��Ҫ��Ϊ__________��ʴ��_____________��ʴ���֡�
��2��������ʴ�������ɿ쵽����˳����_________________(�����)��
��3�����������ĵ缫��ӦʽΪ_________________________________________________�����и����ĵ缫��ӦʽΪ_________________________________________��
��4����������ʾ,ȫ����ÿ����ʴ�����ϵĽ��������൱�����������20%���ϡ�Ϊ������������ʴ�ɲ�ȡ�Ĵ�ʩ��__________(�����)��
�ٽ�������ˢ���� �����г���Ȧ�Ƹ�
�۽��ֹ��õ�����ͭ������ �ܽ��ֹ��õ�����̼������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ϊ��̽����ѧ�������ܵ�ת����ijʵ��С���������ͼ��ʾ������ʵ��װ�ã�
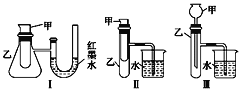
(1)����3��װ���У�����֤����ͭ��Ũ�� �ᷴӦ�����ȷ�Ӧ���Ƿ��ȷ�Ӧ������(�������ִ�����ʹ���¶ȼ�)___________��
(2)ijͬѧѡ��װ�â����ʵ��(ʵ��ǰU�ι���Һ��������ƽ)���ڼ��Թ������ ��������������Һ��ϡ���ᣬU�ι��пɹ۲쵽��������______________��˵���÷�Ӧ����_____ (��������������������)��Ӧ��
(3)Ϊ�����ⶨ(2)�з�Ӧ�ķ�Ӧ�ȣ�ʹ50 mL 0.50 mol��L��1������50 mL0.55 mol��L��1NaOH��Һ�ڼ������ȼ��н����кͷ�Ӧ.�ش��������⣺
�ٸ�ʵ����NaOH��Ũ�ȴ���HCl��Ũ�ȵ�������_________________________��
��ʵ����������60 mL 0.50 mol��L��1������50 mL 0.55 mol��L��1NaOH��Һ���з�Ӧ��������ʵ����ȣ������к���_________ (��������������������)��
(4)��֪һЩ��ѧ���ļ������������ʾ��
��ѧ�� | C��H | C��F | H��F | F��F |
����/kJ��mol��1 | 414 | 489 | 565 | 155 |
����ݼ������ݹ���CH4(g)��F2(g)��Ӧ����CF4(g)��HF(g)���Ȼ�ѧ����ʽ��_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����д�������ĵ���ʽ��
(1)NH4+��____________�� CO2��__________��NaOH��________________ ��
(2)��2.6g��Ȳ��C2H2��̬����ȫȼ������Һ̬ˮ��CO2ʱ����130kJ����˷�Ӧ���Ȼ�ѧ����ʽΪ��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���±��г��ˢ١������Ԫ�������ڱ��е�λ�ã�
��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 | |
1 | �� | |||||||
2 | �� | �� | �� | �� | ||||
3 | �� | �� | �� | �� |
�밴Ҫ��ش��������⡣
��1��Ԫ�آܵ�������______��Ԫ�آ������ڱ�������λ��___________����Ԫ��ԭ�ӵ�ʧ���ӵĽǶȿ���Ԫ�آܾ���____________(������ԡ���ԭ�ԡ�)��
��2������̬�⻯����ȶ���������ǿ��˳�����У��ޢܢߵ��⻯���ȶ��ԣ�_____________________(д�⻯��Ļ�ѧʽ)��
��3��Ԫ�آߵ�ԭ�ӽṹʾ��ͼ��____________��
��4��д��Ԫ�آ��γɵĵ�����������ȼ�յĻ�ѧ����ʽ________________����һ��Ӧ��___________(����ȡ����ȡ�)��Ӧ��
��5���õ���ʽ��ʾ����ᷴӦ�õ��Ļ�������γɹ���_______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ǽ��������Ϊ����ʺͷǵ�������࣬�������ڵ���ʵ���
A. ���� B. ������ C. �Ȼ��� D. ����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com