
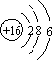 ���ʻ�����ӵĵ���ʽΪ
���ʻ�����ӵĵ���ʽΪ ��
��
���� ��1��S��������Ϊ16��ԭ�Ӻ�����3�����Ӳ㣬��������Ϊ6���ʻ�������������̼���ӽṹ���ƣ���Ϊֱ���ͣ�
��2��a���Ҵ��к������
b��ͬ�¶�ͬŨ����Na2CO3��Һ��pH����Na2SO4��Һ����֪��Ӧ��ۺ���������ԣ�
c��CO2�ṹ�Գƣ�������������غϣ�
d�������һ���Ӱ�죬�����ѵ���������ӣ�
��3���ٷ�ӦI���������������⣬����ˮ���ɣ���Ԫ���غ��֪����������ΪNa2S��Na2CO3��
��������ˮ��Ӧ����S2O32-���������������ƣ����ݵ����غ��ԭ���غ���д��
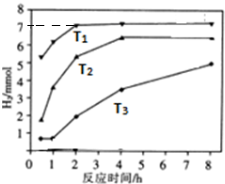
��a����ͼ��֪���¶ȸߵķ�Ӧ���ʴ���Ӧ��ʱ��̣�
b.3molNa2S��ֻ����S2O32-ת��12mol���ӣ�T1�¶��£����ɵ�����Ϊ7mol��ת�Ƶ���Ϊ14mol����ϵ����غ���㣮
��� �⣺��1��S��������Ϊ16��ԭ�Ӻ�����3�����Ӳ㣬��������Ϊ6����ԭ�ӽṹʾ��ͼΪ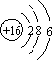 ���ʻ�������������̼���ӽṹ���ƣ���Ϊֱ���ͣ������ʽΪ
���ʻ�������������̼���ӽṹ���ƣ���Ϊֱ���ͣ������ʽΪ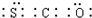 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��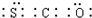 ��
��
��2��a���Ҵ��к���������Ҵ��е����������a����
b��ͬ�¶�ͬŨ����Na2CO3��Һ��pH����Na2SO4��Һ����֪��Ӧ��ۺ����������Ϊ���̼�ᣬ����Ԫ�طǽ�����ǿ��̼Ԫ�أ���b��ȷ��
c��CO2�ṹ�Գƣ�������������غϣ���Ϊ�Ǽ��Է��ӣ�Ϊֱ�߽ṹ����H2S����V�ͼ��Է��ӣ���c����
d�������һ���Ӱ�죬�����ѵ���������ӣ�������������H2S����d��ȷ��
�ʴ�Ϊ��bd��
��3���ٷ�ӦI���������������⣬����ˮ���ɣ���Ԫ���غ��֪����������ΪNa2S��Na2CO3����ӦΪCOS+4NaOH=Na2S+Na2CO3+2H2O��
�ʴ�Ϊ��COS+4NaOH=Na2S+Na2CO3+2H2O��
��������ˮ��Ӧ����S2O32-���������������ƣ��䷴Ӧ�����ӷ���ʽΪ��2S2-+5H2O=S2O32-+4H2��+2OH-���ʴ�Ϊ��2S2-+5H2O=S2O32-+4H2��+2OH-��
��a����ͼ��֪���¶ȸߵķ�Ӧ���ʴ���Ӧ��ʱ��̣���T1��T2��T3���ʴ�Ϊ��T1��T2��T3��
b.3molNa2S��ֻ����S2O32-ת��12mol���ӣ�T1�¶��£����ɵ�����Ϊ7mol��ת�Ƶ���Ϊ14mol���������SO42-Ϊx���ɵ����غ��֪x��8+��3-x����4=14�����x=0.5mol����n��S2O32-��=$\frac{3-0.5}{2}$=1.25mol����Һ��c��S2O32-����c��SO42-��=1.25��0.5=5��2��
�ʴ�Ϊ��5��2��
���� ���⿼����ۺϣ��漰���ʽṹ�����ʡ�������ԭ��Ӧ���㡢ͼ����������ӷ�Ӧ�ȣ����ط�Ӧԭ���и�Ƶ����Ŀ��飬�ۺ��Խ�ǿ����Ŀ�ѶȲ���


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
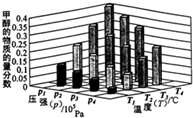
| A�� | P3��P2 T3��T2 | B�� | P2��P4 T4��T2 | C�� | P1��P3 T3��T1 | D�� | P1��P4 T2��T3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
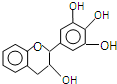 ���豶���������к��в��ӣ�����ûʳ�Ӷ����أ�EGC���Ľṹ��ͼ��ʾ������EGC��������������ȷ���ǣ�������
���豶���������к��в��ӣ�����ûʳ�Ӷ����أ�EGC���Ľṹ��ͼ��ʾ������EGC��������������ȷ���ǣ�������| A�� | ���л���ķ���ʽ��C15H13O5 | |
| B�� | 1mol EGC��4molNa�����������Ϊ44.8L | |
| C�� | ����������Ӧ��ȡ����Ӧ���ѷ����ӳɷ�Ӧ | |
| D�� | ���������е�ԭ�ӹ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
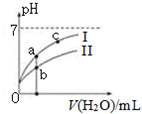 ��֪���±�Ϊ25��ʱijЩ����ĵ���ƽ�ⳣ����
��֪���±�Ϊ25��ʱijЩ����ĵ���ƽ�ⳣ����| CH3COOH | HClO | H2CO3 |
| Ka=1.8��10-5 | Ka=3.0��10-8 | Ka1=4.4��10-7Ka2=4.7��10-11 |
| A�� | ��ͬŨ�ȵ�CH3COONa��NaClO�Ļ����Һ�У�������Ũ�ȵĴ�С��ϵ�ǣ�c��Na+����c��ClO-����c��CH3COO-����c��OH-����c��H+�� | |
| B�� | ��NaClO��Һ��ͨ������������̼�����ӷ���ʽΪ��ClO-+CO2+H2O=HClO+CO32- | |
| C�� | ͼ����a��c���㴦����Һ��$\frac{c��{R}^{-}��}{c��HR��•c��O{H}^{-}��}$��ȣ�HR����CH3COOH��HClO�� | |
| D�� | ͼ����a�������Ũ�ȴ���b�������Ũ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 �״���һ����Ҫ�Ļ���ԭ�ϣ�
�״���һ����Ҫ�Ļ���ԭ�ϣ�| CO2%-CO%-H2% ����������� | 0-30-70 | 2-28-70 | 4-26-70 | 8-22-70 | ||||||||
| ��Ӧ�¶�/�� | 225 | 235 | 250 | 225 | 235 | 250 | 225 | 235 | 250 | 225 | 235 | 250 |
| ����CH3OH��̼ת���ʣ�%�� | 4.9 | 8.8 | 11.0 | 36.5 | 50.7 | 68.3 | 19.0 | 33.1 | 56.5 | 17.7 | 33.4 | 54.4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
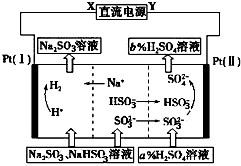 ��Na2SO3��Һ�������Ṥҵβ���еĶ����������õĻ��Һ���е��ѭ�������������¹��ս�����ѭ�������������������ӽ���Ĥ���ѭ������������ͼ��ʾ���������й�˵���в���ȷ���ǣ�������
��Na2SO3��Һ�������Ṥҵβ���еĶ����������õĻ��Һ���е��ѭ�������������¹��ս�����ѭ�������������������ӽ���Ĥ���ѭ������������ͼ��ʾ���������й�˵���в���ȷ���ǣ�������| A�� | XΪֱ����Դ�ĸ�����YΪֱ����Դ������ | |
| B�� | ������pH���� | |
| C�� | ͼ�е�b��a | |
| D�� | �ù����еIJ�Ʒ����H2SO4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ʹ�����Ժ�ɫ����Һ�д������ڣ�Mg2+��Na+��Cl-��F- | |
| B�� | ��״���£�46gNO2��N2O4��������к���ԭ�Ӹ���Ϊ3NA | |
| C�� | 1L0.5mol•L-1 CuSO4��Һ�к���0.5NA��Cu2+ | |
| D�� | Ũ�Ⱦ�Ϊ0.1 mol/L�İ�ˮ�����ᡢ��ˮ�������c��H+���������ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ij�ֺ��ص����ԭ������ | |
| B�� | ij�ֺ��ص������� | |
| C�� | ij��Ԫ�����к�����������ƽ��ֵ | |
| D�� | ij��Ԫ�ص�ƽ�����ԭ�������Ľ���ֵ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com