
Ԫ�����ڱ��е�������Ԫ��������3d���ӵ�Ӱ�죬���ʵĵݱ�����������Ԫ�����в�ͬ��
(1)�������ڹ���Ԫ�ص������������γɶ��ֶ���������
��CO���Ժͺܶ���ɽ����γ�����CO������Cԭ������һ�Թ¶Ե��ӣ�C��Oԭ�Ӷ�����8�����ȶ��ṹ��CO�ĽṹʽΪ________��CO������Ĺ��ۼ���________��
�ڽ���������CO���������ؼ��ȣ�������Һ̬��Ni(CO)4������423K�ͷֽ�ΪNi��CO���Ӷ��Ƶøߴ��ȵ�Ni�ۣ�Ni(CO)4����������________��
a��ˮ��b�����Ȼ�̼��c������d����������Һ
(2)��������Ԫ�صĵ�һ��������ԭ��������������������������ģ�
�صĻ�̬�����Ų�ʽ��________��
31
Ga�ĵ�һ������ȴ���Ե���30Zn��ԭ����________��
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
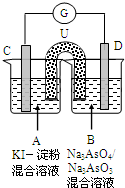 ��ͼ��һ�绯ѧʵ��װ�ã�ͼ��C��D��Ϊ���缫��UΪ���ţ�G�����������ƣ���ָ������ƫ���Դ������
��ͼ��һ�绯ѧʵ��װ�ã�ͼ��C��D��Ϊ���缫��UΪ���ţ�G�����������ƣ���ָ������ƫ���Դ������| [H+]2?[I-]2?[AsO43-] |
| [I2]?[AsO33-] |
| [H+]2?[I-]2?[AsO43-] |
| [I2]?[AsO33-] |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com