
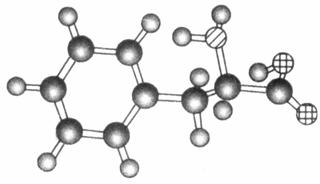
��������������������̥���ϳɶ�����һ�ֵ���A�ķ���ʽΪC4H8��A�⻯��õ�2�������顣
���������գ�
(1)A���Ծۺϣ�д��A�����־ۺϷ�ʽ(�Է�Ӧ����ʽ��ʾ)��______________________________________________________________________________________________________________________
(2)A��ij�鷢���������Ӧ���ɷ���ʽΪC8H18������B��B��һ±����ֻ��4�֣���̼�����Գơ�д��B�Ľṹ��ʽ��________________________________________________________________________________________________________________________________________________��
(3)д����Aͨ������������Һ����ֵ�����
Aͨ����ˮ��__________________________________ ______________________________________��
Aͨ��������Ȼ�̼��Һ��________________________________ _________________________________��
(4)ϩ����NBS���ã�ϩ������˫��̼����̼ԭ���ϵ�һ����ԭ�ӱ���ԭ��ȡ��������ʽΪC4H8������NBS���ã��õ���һ���ϩ����________�֡�

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij���ʵ����ģ����ͼ��ʾ�����ڸ����ʵ�˵������ȷ����(����)
A�������ʵĽṹ��ʽΪ
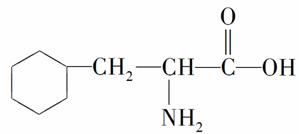
B�������ʿ��Է����ӳɷ�Ӧ
C�������ʼȿ�����ǿ�ᷴӦҲ������ǿ�Ӧ
D�������ʿ��Ծۺϳɸ߷�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������������Һ������������ԭ��Ӧ�����ܴ���������ǣ�������
A��Ag+��NO3����Cl����K+ B��H+��NO3����Fe2+��Na+
C��K+��Ba2+��OH����SO42�� D��Cu2+��NH4+��Br����OH��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ж����л��߷��ӻ�����������������(����)
A���߷��ӻ�����ɷ�Ϊ��Ȼ�߷��ӻ�����ͺϳɸ߷��ӻ�����������
B���߷��ӻ�������ص�֮һ�����Ԫ�ؼ��ṹ���ӡ���Է���������
C���߷��ӻ������Ϊ�����
D���ϳɵ��л��߷��ӻ����������С���ӻ�����ͨ���ۺϷ�Ӧ���Ƶõ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵������ȷ����(����)
A���߷��ӻ��������һ�㶼�й̶����۵�
B����Է���������ĸ߷���һ����������ԣ���Է�������С�ĸ߷���һ������ȹ���
C���߾���ľۺ϶�һ���Dz�ȷ����
D���߷��ӻ�������ܽ�һ��Ƚϻ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ͼ��ʾΪ�������˻�ļ�����������Ϊ����ë���ڳ������ά���þ�����ά�Ľṹ��ʽΪ
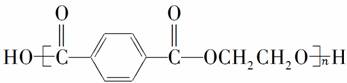 ������˵������ȷ����(����)
������˵������ȷ����(����)
A����ë�������ά�Ļ�ѧ�ɷֲ���ͬ
B���ɵ���ϳɾ�����ά�ķ�Ӧ���ڼӾ۷�Ӧ
C���þ�����ά�ĵ���Ϊ�Ա���������Ҷ���
D��������ά����ë��һ�������¾���ˮ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���и����Ȼ�ѧ����ʽ�У���ѧ��Ӧ�Ħ�Hǰ�ߴ��ں��ߵ���(����)
��C(s)��O2(g)===CO2(g)����H1
C(s) ��
��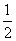 O2(g)===CO(g)����H2
O2(g)===CO(g)����H2
 ��S(s)��O2(g)===SO2(g)����H3
��S(s)��O2(g)===SO2(g)����H3
S(g)��O2(g)===SO2(g)����H4
��H2(g)��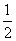 O2(g)===H2O(l)����H5
O2(g)===H2O(l)����H5
2H2(g)��O2(g)===2H2O(l)����H6
��CaCO3(s)===CaO(s)��CO2(g)����H7
CaO(s)��H2O(l)===Ca(OH)2(s)����H8
A���� B����
C���ڢۢ� D���٢ڢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ϣ���������ϡ��͡���Դ������Ϊ�¿Ƽ�����������֧���������й���Ѷ�������(����)
A���ڼ�������������Դʱ�������ܡ�̫���ܡ����ܽ���Ϊ��Ҫ��Դ
B��Ŀǰ�����յȹ����յ��մɷ������������������˷�����������ת��Ч��
C��2002��12��30�շ���ɹ��ġ������ĺš���ʹ���˴����ĸ��ϲ���
D���ϳɸ߷��Ӳ��ϵĹ㷺Ӧ���ǡ��а�������һ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com