

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 ��ҵ��ˮ�Ĵ�����
��ҵ��ˮ�Ĵ�����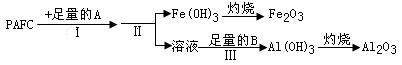
 �����⣺
�����⣺
 ��Һ�м����Լ�X��Ŀ����_________________________________��
��Һ�м����Լ�X��Ŀ����_________________________________���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 ����ţ���
����ţ���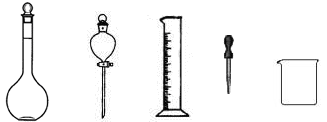
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
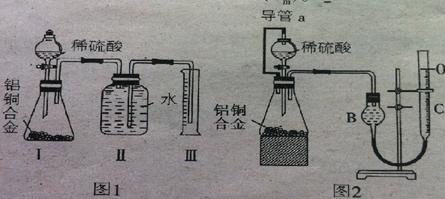
| | ʵ��ǰ | ʵ��� |
| ��ͭ�Ͻ�������g�� | m1 | m2 |
| ��Һ�ܣ�C�������mL�� | V1 | V2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 2��NO������֤�����ʣ�װ��ͼ���£�
2��NO������֤�����ʣ�װ��ͼ���£�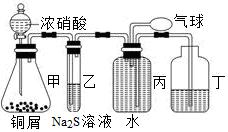
 ��)��
��)���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 ��AlN���Ʊ���Al2O3+N2+3C
��AlN���Ʊ���Al2O3+N2+3C 2AlN+3CO����Ӧ�����������뻹ԭ��������ʵ���֮��Ϊ ��
2AlN+3CO����Ӧ�����������뻹ԭ��������ʵ���֮��Ϊ �� CO2+2H2O+4Cu��Ϊ�ⶨ��Ʒ���йسɷֵĺ����������������£�
CO2+2H2O+4Cu��Ϊ�ⶨ��Ʒ���йسɷֵĺ����������������£�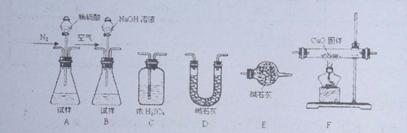
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
A��ϡ���� | B����ɫʯ����Һ | C��ˮ | D������������Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������ƿ��ԭ����������ˮ | B������ʱ���ӿ̶��� |
| C��������NaOH��Һ�������ձ��� | D������ʱ�����Ϊ��������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��п����ϡHNO3��Ӧ�Ʊ�H2 |
| B��������ڵ�Al2O3�Ʊ�O2 |
| C������MnO2��ŨHCl��Ӧ�Ʊ�Cl2 |
| D������ʯ��ŨH2SO4��Ӧ�Ʊ�CO2 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com