
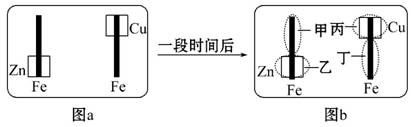
ŅŃÖŖĒ¦Šīµē³ŲµÄ¹¤×÷ŌĄķĪŖPb+PbO2+2H2SO4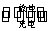
2PbSO4+2H2O,ĻÖÓĆČēĶ¼×°ÖĆ½ųŠŠµē½ā(µē½āŅŗ×ćĮæ),²āµĆµ±Ē¦Šīµē³ŲÖŠ×ŖŅĘ
0.4 molµē×ÓŹ±Ģśµē¼«µÄÖŹĮæ¼õÉŁ11.2 g”£Ēė»Ų“šĻĀĮŠĪŹĢā:

(1)AŹĒĒ¦Šīµē³ŲµÄ””””””””¼«,Ē¦Šīµē³ŲÕż¼«·“Ó¦Ź½ĪŖ””””””””””””””””””,·Åµē¹ż³ĢÖŠµē½āŅŗµÄĆܶȔ”””””””(Ģī”°¼õŠ””±”°Ōö“ó”±»ņ”°²»±ä”±)”£
(2)Agµē¼«µÄµē¼«·“Ó¦Ź½ŹĒ””””””””””””””””,øƵē¼«µÄµē¼«²śĪļ¹²””””””””g”£
(3)Cuµē¼«µÄµē¼«·“Ó¦Ź½ŹĒ””””””””””””””””””””,CuSO4ČÜŅŗµÄÅØ¶Č””””””””(Ģī”°¼õŠ””±”°Ōö“ó”±»ņ”°²»±ä”±)”£
(4)ČēĶ¼±ķŹ¾µē½ā½ųŠŠ¹ż³Ģ֊ijøöĮæ(ׯ×ų±źx)Ėꏱ¼äµÄ±ä»ÆĒśĻß,Ōņx±ķŹ¾”””””””””£
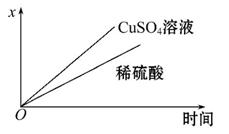
a.ø÷UŠĪ¹ÜÖŠ²śÉśµÄĘųĢåµÄĢå»ż
b.ø÷UŠĪ¹ÜÖŠŃō¼«ÖŹĮæµÄ¼õÉŁĮæ
c.ø÷UŠĪ¹ÜÖŠŅõ¼«ÖŹĮæµÄŌö¼ÓĮæ
øł¾ŻŌŚµē½ā¹ż³ĢÖŠĢśµē¼«ÖŹĮæµÄ¼õÉŁæÉÅŠ¶ĻAŹĒµēŌ“µÄøŗ¼«,BŹĒµēŌ“µÄÕż¼«,µē½āŹ±Ag¼«×÷Ņõ¼«,µē¼«·“Ó¦Ź½ĪŖ2H++2e-====H2”ü,Fe×÷Ńō¼«,µē¼«·“Ó¦Ź½ĪŖFe-2e-====Fe2+,×ó²ąUŠĪ¹ÜÖŠ×Ü·“Ó¦Ź½ĪŖFe+2H+====Fe2++H2”ü”£ÓŅ²ąUŠĪ¹ÜĻąµ±ÓŚµē¶Ę×°ÖĆ,Znµē¼«×÷Ņõ¼«,µē¼«·“Ó¦Ź½ĪŖCu2++2e-====Cu,Ķµē¼«×÷Ńō¼«,µē¼«·“Ó¦Ź½ĪŖCu-2e-====Cu2+,µē¶Ę¹ż³ĢÖŠCuSO4ČÜŅŗµÄÅØ¶Č±£³Ö²»±ä,øł¾ŻÉĻŹö·ÖĪöæɵƓš°ø”£
“š°ø:(1)øŗ””PbO2+4H++S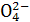 +2e-====PbSO4+2H2O””¼õŠ”
+2e-====PbSO4+2H2O””¼õŠ”
(2)2H++2e-====H2”ü””0.4
(3)Cu-2e-====Cu2+””²»±ä””(4)b
”¾·½·Ø¼¼ĒÉ”æµē½ā¼ĘĖćµÄŅ»°ć½āĢāĖ¼Ā·
(1)ĻČæ“Ńō¼«²ÄĮĻŹĒ»īŠŌµē¼«»¹ŹĒ¶čŠŌµē¼«”£
(2)ÕŅČ«Ąė×Ó,½«ČÜŅŗÖŠµÄĖłÓŠĄė×Ó°“ŅõŃōĄė×Ó·ÖæŖ”£
(3)øł¾Żµē×ÓŹŲŗć,“®ĮŖµēĀ·ÖŠ×ŖŅʵĵē×ÓŹżÄæĻąĶ¬,¼“ŅõĄė×Ó(»īĘƵē¼«)Ź§Č„µÄµē×ÓŹżµČÓŚŃōĄė×ӵƵ½µÄµē×ÓŹż,ĮŠ³ö¹ŲĻµŹ½½ųŠŠ¼ĘĖć”£³£ÓĆµÄ¼ĘĖć¹ŲĻµŹ½ÓŠ:4H+”«4OH-”«2H2”«O2”«2Cu2+”«2Cu”«4Ag”«4Ag+”«4Cl-”«2Cl2µČ”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ÓĆČēĶ¼×°ÖĆ½ųŠŠĢś·ŪŌŚøßĪĀĻĀÓėĖ®ÕōĘų·“Ó¦µÄŹµŃ飬²¢ÓĆ¼ņµ„µÄ·½·ØŹÕ¼Æ”¢¼ģŃéÉś³ÉµÄĒāĘų”£Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
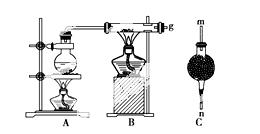
£Ø1£©Š“³öĢśŌŚøßĪĀĻĀÓėĖ®ÕōĘų·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ _______________________________”£
£Ø2£©øÉŌļ¹ÜCÄŚŹ¢·ÅµÄŅ©Ę·ŹĒ________”£øÉŌļ¹ÜµÄ________(Ģī”°m”±»ņ”°n”±)¶ĖÓėµ¼¹ÜæŚ g ĻąĮ¬½Ó”£
£Ø3£©ČōŹÕ¼Æµ½±ź×¼×“æöĻĀµÄH2ĪŖ22.4 L£¬Ōņ²Ī¼Ó·“Ó¦µÄĢś·ŪµÄÖŹĮæĪŖ________g”£
£Ø4£©µ±¹ĢĢåÖŹĮæŌö¼Ó32 gŹ±£¬Éś³ÉH2µÄÖŹĮæĪŖ________g”£
gŹ±£¬Éś³ÉH2µÄÖŹĮæĪŖ________g”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
½«CuÓėCuOµÄ»ģŗĻĪļ20.8g¼ÓČėµ½50mL 18.4mol/LÅØH2SO4ÖŠ£¬¼ÓČČ³ä·Ö·“Ó¦ÖĮ¹ĢĢåĪļÖŹĶźČ«Čܽā(²śÉśĘųĢåČ«²æŅŻ³ö)£¬ĄäČ“ŗó½«ČÜŅŗĻ”ŹĶÖĮ1000ml£¬²āµĆc(H+)=0.84mol/L£»ČōŅŖŹ¹Ļ”ŹĶŗóČÜŅŗÖŠµÄCu2+³ĮµķĶźČ«£¬Ó¦¼ÓČė6.0mol /LµÄNaOHČÜŅŗµÄĢå»żĪŖ
/LµÄNaOHČÜŅŗµÄĢå»żĪŖ
A£® 100mL B£® 160mL C£® 240mL D£® 307mL
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻÖÓŠČēĻĀø÷»ÆŗĻĪļ£ŗ¢Ł¾Ę¾«£¬¢ŚĀČ»Æļ§£¬¢ŪĒāŃõ»Æ±µ£¬¢Ü°±Ė®£¬¢ŻÕįĢĒ£¬¢ŽøßĀČĖį£¬¢ßĒāĮņĖį£¬¢ąĮņĖįĒā¼Ų£¬¢įĮ×Ėį£¬¢āĮņĖį”£
ĒėÓĆĪļÖŹµÄŠņŗÅĢīŠ“ĻĀĮŠæÕ°×£ŗ
(1)ŹōÓŚµē½āÖŹµÄÓŠ
________________________________________________________________________ӣ
(2)ŹōÓŚĒæµē½āÖŹµÄÓŠ
________________________________________________________________________ӣ
(3)ŹōÓŚČõµē½āÖŹµÄÓŠ
________________________________________________________________________ӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ijŠ£»ī¶ÆŠ”×éĪŖĢ½¾æ½šŹōøÆŹ“µÄĻą¹ŲŌĄķ,Éč¼ĘĮĖČēĶ¼aĖłŹ¾×°ÖĆ,Ķ¼aµÄĢś°ōÄ©¶Ī·Ö±šĮ¬ÉĻŅ»æéZnʬŗĶCuʬ,²¢ÖĆÓŚŗ¬ÓŠK3Fe(CN)6¼°·ÓĢŖµÄ»ģŗĻÄż½ŗÉĻ”£Ņ»¶ĪŹ±¼äŗó·¢ĻÖÄż½ŗµÄijŠ©ĒųÓņ(ČēĶ¼bĖłŹ¾)·¢ÉśĮĖ±ä»Æ,ŅŃÖŖFe2+æÉÓĆK3Fe(CN)6Ą“¼ģŃé(³ŹĄ¶É«)”£ŌņĻĀĮŠĖµ·Ø²»ÕżČ·µÄŹĒ(””””)
A.¼×Ēų·¢ÉśµÄµē¼«·“Ó¦Ź½:Fe-2e-====Fe2+
B.ŅŅĒų²śÉśZn2+
C.±ūĒų³ŹĻÖŗģÉ«
D.¶”Ēų³ŹĻÖĄ¶É«
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
¹¤ŅµŗĻ³É°±µÄ·“Ó¦ŹĒŌŚ500”ę×óÓŅ½ųŠŠµÄ£¬ÕāÖ÷ŅŖŹĒŅņĪŖ(””””)
A£®500”ꏱ“Ė·“Ó¦ĖŁĀŹ×īæģ
B£®500”ꏱNH3µÄĘ½ŗāÅضČ×ī“ó
C£®500”ꏱN2µÄ×Ŗ»ÆĀŹ×īøß
D£®500”ꏱøĆ·“Ó¦µÄ“߻ƼĮ»īŠŌ×ī“ó
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
øł¾ŻÅöײĄķĀŪ£¬·Ö×ÓŌŚ·¢Éś·“Ó¦Ź±±ŲŠėŅŖ½ųŠŠÓŠŠ§Åöײ”£ÄĒŠ©¾ßÓŠ×ć¹»øßÄÜĮ棬ÄÜ·¢ÉśÓŠŠ§ÅöײµÄ·Ö×Ó³ĘĪŖ»ī»Æ·Ö×Ó£¬ŅŖŹ¹ĘÕĶØ·Ö×Ó³ÉĪŖ»ī»Æ·Ö×ÓĖłŠč×īŠ”ÄÜĮæ³ĘĪŖ»ī»ÆÄÜ(Ea)”£Ņ»¶ØĪĀ¶ČĻĀĘųĢå·Ö×ÓÖŠµÄ»ī»Æ·Ö×Ó°Ł·ÖŹżŹĒŅ»¶ØµÄ£¬¶ų“߻ƼĮæÉŅŌøıä»ī»ÆÄܵē󊔔£ČēĶ¼±ķŹ¾298.15 KŹ±£¬N2”¢H2ÓėNH3µÄĘ½¾łÄÜĮæÓėŗĻ³É°±·“Ó¦µÄ»ī»ÆÄܵÄĒśĻßĶ¼£¬¾ŻĶ¼»Ų“š£ŗ
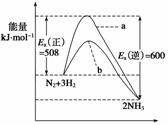
(1)Čō·“Ó¦ÖŠÉś³É2 mol°±£¬Ōņ·“Ó¦________(Ģī”°ĪüČČ”±»ņ”°·ÅČČ”±)________kJ”£
(2)ŌŚĶ¼ÖŠĒśĻß________(Ģī”°a”±»ņ”°b”±)±ķŹ¾¼ÓČėĢś“„Ć½µÄÄÜĮæ±ä»ÆĒśĻߣ¬Ģś“„Ć½ÄܼÓæģ·“Ó¦ĖŁĀŹµÄŌĄķŹĒ_____________________________________________________________
________________________________________________________________________ӣ
(3)ÄæĒ°ŗĻ³É°±¹¤Ņµ¹ć·ŗ²ÉÓƵķ“Ó¦Ģõ¼ž500”ę”¢20 MPa”«50 MPa”¢Ģś“„Ć½£¬·“Ó¦×Ŗ»ÆĀŹ²»³¬¹ż50%£¬¹¤ŅµÉĻĪŖĮĖ½ųŅ»²½Ģįøß°±Ęų²śĀŹ£¬ÄćČĻĪŖĻĀĮŠ“ėŹ©×ī¾¼ĆæÉŠŠµÄŹĒ
________________________________________________________________________ӣ
A£®½µµĶ·“Ó¦ĪĀ¶Č£¬ČĆ·“Ó¦Ļņ×ÅÓŠĄūÓŚ°±ĘųÉś³ÉµÄ·½Ļņ½ųŠŠ
B£®ÉżøßĪĀ¶Č£¬ČĆøü¶ąµÄ·Ö×Ó±ä³É»ī»Æ·Ö×Ó
C£®Ń°ĒóÄÜŌŚøüµĶµÄĪĀ¶ČĻĀÓŠŗÜĒæ“߻ƻīŠŌµÄŠĀŠĶ“߻ƼĮ
D£®Ń°ĒóŠĀŠĶÄĶøßŃ¹²ÄĮĻ£¬½«Ń¹ĒæŌö“óŅ»±¶
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Mg(OH)2ÄŃČÜÓŚĖ®£¬µ«ĖüČܽāµÄ²æ·ÖČ«²æµēĄė”£ŹŅĪĀĻĀŹ±£¬±„ŗĶMg(OH)2ČÜŅŗµÄpH£½11£¬Čō²»æ¼ĀĒKWµÄ±ä»Æ£¬ŌņøĆĪĀ¶ČĻĀMg(OH)2µÄČܽā¶ČŹĒ¶ąÉŁ£æ(ČÜŅŗĆܶČĪŖ1.0 g”¤cm£3)
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠø÷ÖÖĒéæöĻĀŅ»¶ØÄÜ“óĮæ¹²“ęµÄĄė×Ó×éĪŖ(””””)
A£®pH£½7µÄČÜŅŗÖŠ£ŗFe3£«”¢Cl£”¢Na£«”¢NO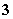
B£®Ė®µēĄė³öµÄ[H£«]£½1”Į10£3 mol”¤L£1µÄĖ®ČÜŅŗÖŠ£ŗNa£«”¢CO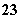 ”¢Cl£”¢K£«
”¢Cl£”¢K£«
C£®pH£½1µÄĖ®ČÜŅŗÖŠ£ŗNH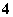 ”¢Cl£”¢Mg2£«”¢SO
”¢Cl£”¢Mg2£«”¢SO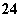
D£®Al3£«”¢HCO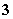 ”¢I£”¢Ca2£«
”¢I£”¢Ca2£«
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com