
ij�о���ѧϰС������ͭм������ͭ������������������ɵĻ��ᷴӦ����ȡCuSO4��5H2O���壬����������Ļ�ԭ����ΪNO����Ӧ�����в�����SO2,��Ӧ�����Һ�в���Cu(NO3)2����Ӧ�й�����ȫ�ܽ⣬�������ǡ����ȫ��Ӧ������������������Ϊ480 g������ͭ����������Ϊx���Իش����С�
��1����������ͭ���������yΪ�� g����x��ʾ����
��2����x =0.4,������HNO3��H2SO4�����ʵ���֮��Ϊ�� ��
��3����x=0.4,480g����������һ���������Ⱥ�ַ�Ӧ����ȴǡ��ֻ�õ�CuSO4��5H2O,����ԭ������H2SO4������������

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��1������֬�ް�סԼ0.2g�������Ʒ�ĩ������ʯ�����ϣ�����֬���ϵ�ˮ���ɹ۲쵽��֬����ȼ����������ʵ���������ó����й�Na2O2��H2O��Ӧ�Ľ����ǣ�
��1������֬�ް�סԼ0.2g�������Ʒ�ĩ������ʯ�����ϣ�����֬���ϵ�ˮ���ɹ۲쵽��֬����ȼ����������ʵ���������ó����й�Na2O2��H2O��Ӧ�Ľ����ǣ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
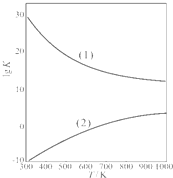 ��2011?ɽ��ģ�⣩��ȩ����Ҫ���л��ϳ�ԭ�ϣ���������������֬���ϳ���ά��ҩ�Ϳ�ϵȣ�Ҳ������������֯��WHO��ȷ�ϵ��°�����»�����֮һ�����й����ڻ�������ίԱ�����ͳ�ƣ��й���װ��ͥ��ȩ����60%���ϣ��ҹ��涨���ڿ����м�ȩ�������ó���0.08mg/m3��
��2011?ɽ��ģ�⣩��ȩ����Ҫ���л��ϳ�ԭ�ϣ���������������֬���ϳ���ά��ҩ�Ϳ�ϵȣ�Ҳ������������֯��WHO��ȷ�ϵ��°�����»�����֮һ�����й����ڻ�������ίԱ�����ͳ�ƣ��й���װ��ͥ��ȩ����60%���ϣ��ҹ��涨���ڿ����м�ȩ�������ó���0.08mg/m3��| 1 | 2 |
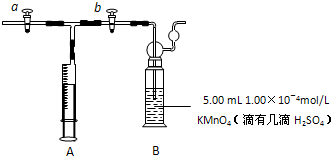
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
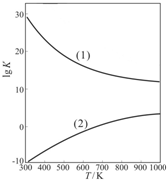
| 1 | 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
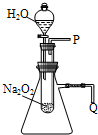 ����֬�ް�סԼ0.2g�������Ʒ�ĩ������ʯ�����ϣ�����֬���ϵ�ˮ���۲쵽��֬����ȼ��������
����֬�ް�סԼ0.2g�������Ʒ�ĩ������ʯ�����ϣ�����֬���ϵ�ˮ���۲쵽��֬����ȼ���������鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com