
| A�� | �ۢݢ� | B�� | �٢ڢۢ� | C�� | �٢ڢܢ� | D�� | �٢ڢ� |
���� ��NO2��N2O4�����ʽ��ͬΪNO2������46g NO2������ԭ������
�ڼ���CH4�����ʵ�����Ȼ�����1mol�����к�4��C-H���ۼ���������
����������Ũ��Խ��������ܶ�Խ����10mL��������Ϊ98%��H2SO4����ˮ��100mL��H2SO4��������������9.8%��
�ܱ�״�������Ȼ�̼�������壻
��NaClO��Һ�У����������Ӷ���ˮ����ģ�25��ʱ��pH=12��1.0L NaClO��Һ��������Ũ��Ϊ10-12mol/L������������Ũ��Ϊ0.01mol/L���ݴ˼��㣻
����Һ�����֪���ܼ���������
�߹�����������Ԫ��Ϊ-1�ۣ�1mol Na2O2��ˮ��ȫ��Ӧ����0.5mol������ת����1mol���ӣ�
��� �⣺��NO2��N2O4�����ʽ��ͬΪNO2������46g NO2������ԭ����=$\frac{46g}{46g/mol}$��3��NA=3NA���ʢ���ȷ��
��4gCH4�����ʵ���=$\frac{4g}{16g/mol}$=0.25mol����1mol�����к�4��C-H���ۼ�����0.25mol�����к�1molC-H������NA�����ʢ���ȷ��
���������������Խ��������Һ���ܶ�Խ��ϡ������������������䣬����ϡ�ͺ�������ܶȼ�С����10mL��������Ϊ98%��H2SO4����ˮ��100mL��H2SO4��������������ڣ�$\frac{10}{100}$��98%=9.8%���ʢ۴���
�ܱ�״�������Ȼ�̼�������壬5.6L���Ȼ�̼���ʵ�������0.25mol���ʢܴ���
��25��ʱ��pH=12��1.0L CH3COONa��Һ������������Ũ��Ϊ0.01mol/L����Һ�е�������������ˮ����ģ���1L�ô�������Һ��ˮ����������������ӵ����ʵ���Ϊ0.01mol��OH-����ĿΪ0.01NA���ʢ���ȷ��
��0��lmol•L-1Na2CO3��Һ��̼�������ˮ�⣬��Һ��̼�������Ũ��С��0.1mol/L����Һ�����֪���ܼ���̼������������ʢ���
��1mol Na2O2��ˮ��ȫ��Ӧ����0.5mol������ת����1mol���ӣ�ת�Ƶ�����ΪNA���ʢߴ���
��ѡD��
���� ���⿼�鰢���ӵ���������ؼ��㣬��Ŀ�ѶȲ���ע�����ʵ���ɡ��ṹ�������Լ����ʴ��ڵ���������;ۼ�״̬�����⣮


 �Ǽ�����������ϵ�д�
�Ǽ�����������ϵ�д� â���̸������Ծ�ϵ�д�
â���̸������Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | v��D��=0.4 mol/�� L•S �� | B�� | v��C��=30 mol/�� L•min �� | ||
| C�� | v��B��=36 mol/�� L•min �� | D�� | v��A��=0.15 mol/�� L•S �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��25�桢1.01��105Pa�������£�2.24 L H2�к��еķ�����С��0.1NA | |
| B�� | 1 L 1 mol•L-1��CaCl2��Һ�к�Cl-����ĿΪNA | |
| C�� | �ڱ�״���£�22.4 L H2O������ԼΪ18 g | |
| D�� | 22 g CO2���״����11.2 L H2O������ͬ�ķ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
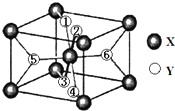 ��Ԫ�����ڱ��У���ϡ�������⼸������Ԫ�ض��������γ��⻯��⻯�ᄃ��Ľṹ�й����ͺ�������֮�֣�
��Ԫ�����ڱ��У���ϡ�������⼸������Ԫ�ض��������γ��⻯��⻯�ᄃ��Ľṹ�й����ͺ�������֮�֣��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NH4+��K+��Ca2+��Cl- | B�� | SO42-��Na+��Fe2+��Cl- | ||
| C�� | Na+��K+��Cl-��SiO32- | D�� | SO42-��NO3-��K+��Na+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��15.6 g Na2O2��5.4 g Alͬʱ����һ������ˮ�п��Բ������������6.72 L | |
| B�� | ij��Һ�м��������ܲ���ʹ����ʯ��ˮ����ǵ����壬��������ʹƷ����Һ��ɫ�������Һ���ܼȺ���SO32-�ֺ���CO32- | |
| C�� | �����̼�Ļ����ﶼ�ǹ��ۻ������ԭ����̼Ԫ��������ͬλ�� | |
| D�� | ʯ�ͷ���ɻ����ϩ����ϩ�Ͷ���ϩ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ͭ˿�������״���ھƾ����ϼ��ȱ�ں���������ʢ����ˮ�Ҵ����Թ��У�ʵ���Ҵ�������Ϊ��ȩ��ʵ�� | |
| B�� | ��Է���������ͬ�IJ�ͬ����һ����ͬ���칹�� | |
| C�� | ���ۺ���ά�صķ���ʽ��ͬ����������Ϊͬ���칹�� | |
| D�� | ������ˮ�������������Ҵ������Ȼ�̼�����л��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
 ���ǰ�ɫ��ɫ��ĩ����ͨ������ʵ�鲽���Ƶã�
���ǰ�ɫ��ɫ��ĩ����ͨ������ʵ�鲽���Ƶã�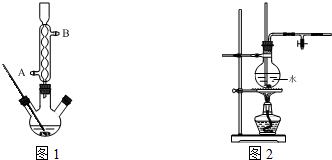
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CH4+Cl2$\stackrel{����}{��}$CH3Cl+HCl | |
| B�� | C6H6ʮBr2$\stackrel{Fe}{��}$C6H5Br+HBr | |
| C�� | CH2�TCH2+Br2CH2Br-CH2Br | |
| D�� | CH3COOH+CH3CH2OH$��_{����}^{Ũ����}$CH3COOCH2CH3+H2O |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com