
������ijЩ����Ԫ�صİ뾶�ͻ��ϼۣ�
| Ԫ�� | N | O | F | P | X | Y |
| ԭ�Ӱ뾶��nm�� | 0.075 | 0.074 | 0.071 | 0.110 | r��X�� | r��Y�� |
| ��ߣ��ͣ����ϼ� | �D3 +5 | �D2 | �D1 | �D3 | �D2 +6 | �D1 +7 |
��1����0.110>r��x��>0.074����XԪ��Ϊ ����Ԫ�ط��ţ���ͬ������r��Y��Ϊ0.114nm����ͨ�������Y����ΪҺ�壬��YԪ��Ϊ ��
��2���Ƚ��ȶ��ԣ�HF HY���>����<�������Ƚ��ȶ��ԣ���������PH3>NH3,��H2S>H2��CY4 CF4�����>����<����
��3����֪XO2����Ư���ԣ�Z��Xͬ���ڡ���Yͬ���壬Z���ʵ�ˮ��ҺҲ����Ư���ԣ��������ʵ�����XO2��Z2ͨ��ˮ�У�����Һ ������С��������С���Ư���ԣ�����Ӧ��������Һ��400mL1mol/LNaOH��Һǡ����ȫ��Ӧ����XO2�����ʵ���Ϊ ��ZԪ�����ϱ��е�PԪ���γɵ�һ�ֻ����������������ԭ�Ӷ�����8�����ȶ��ṹ���û�����Ļ�ѧʽ�� ���˻�������ӵĿռ乹��Ϊ ��
 �㾦�½̲�ȫ�ܽ��ϵ�д�
�㾦�½̲�ȫ�ܽ��ϵ�д� Сѧ�̲���ȫ���ϵ�д�
Сѧ�̲���ȫ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����


| ��� | ���� | ��ѧ����ʽ |
| ʾ�� | ������ | H2WO3+3H3PO3�T3H3PO4+H2W�� |
| 1 | ��ԭ�� ��ԭ�� |
H2SO3+Br2+2H2O=H2SO3+2HBr H2SO3+Br2+2H2O=H2SO3+2HBr |
| 2 | ���� ���� |
H2SO3+2NaOH=Na2SO3+2H2O H2SO3+2NaOH=Na2SO3+2H2O |
| ||
| ����ʯ |
| ||
| ����ʯ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
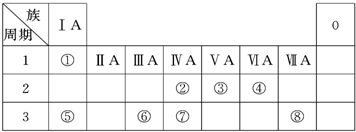
 ������Ԫ��A-F��ԭ����������������Ԫ�ص���Ϣ���£�
������Ԫ��A-F��ԭ����������������Ԫ�ص���Ϣ���£�| Ԫ�ر�� | ��Ϣ |
| B | ��Ԫ��A��D��ͬ��ɵĻ������������� |
| D | �����������Ǵ��������������� |
| E | ͬ����Ԫ�����γɵļ����Ӱ뾶��С |
| F | ����������Ӧ��ˮ�����������ǿ |
| ��� | ���� | ��ѧ����ʽ |
| ʾ�� | ������ | H2WO3+3H3PO3=3H3PO4+H2W�� |
| �� | ||
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(18��)�±�ΪԪ�����ڱ���һ���֣������Ԫ�آ١����ڱ��е�λ�ã��ش��������⣺
(1)�ؿ��к������ڵڶ�λ��Ԫ�������ڱ��е�λ����________________��
(2)�ڡ��ߵ���ۺ��������������ǿ�����ģ���ԭ�ӽṹ����ԭ��
__________��ԭ�Ӱ뾶�����õ��������������ǽ�����������
(3)�١��ܡ��ݡ����е�ijЩԪ�ؿ��γɼȺ����Ӽ��ֺ����ۼ������ӻ����д������һ�ֻ�����ĵ���ʽ_______________��
(4)�ɱ�������Ԫ�ص�ԭ�Ӱ�1��1��ɵij���Һ̬�������ϡ��Һ�ױ����ֽ⣬�������ʲ������÷�Ӧ��������(�����)________��
a��MnO2����b�� CuSO4 c��Na2SO3 d��FeCl3
(5)W��������ڵ�ͬ����Ԫ�ء����±����г�H2WO3�ĸ��ֲ�ͬ��ѧ���ʣ�������д����Ӧ�Ļ�ѧ����ʽ��
| ��� | ���� | ��ѧ����ʽ |
| ʾ�� | ������ | H2WO3��3H3PO3===3H3PO4��H2W�� |
| 1 |
|
|
| 2 |
|
|
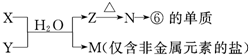
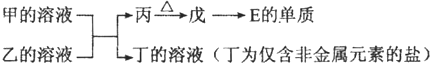
(6)�ɱ���Ԫ���γɵij�������X��Y��Z��M��N�ɷ������·�Ӧ��
X��Һ��Y��Һ��Ӧ�����ӷ���ʽ______________��
N���ĵ��ʵĻ�ѧ����ʽΪ____________��
M��Һ������Ũ���ɴ�С������˳����______________ ��
M�������ӵļ������� __________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�걱����ʯ��ɽ��������ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ������
(18��)�±�ΪԪ�����ڱ���һ���֣������Ԫ�آ١����ڱ��е�λ�ã��ش��������⣺
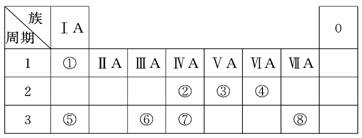
(1)�ؿ��к������ڵڶ�λ��Ԫ�������ڱ��е�λ����________________��
(2)�ڡ��ߵ���ۺ��������������ǿ�����ģ���ԭ�ӽṹ����ԭ��
__________��ԭ�Ӱ뾶�����õ��������������ǽ�����������
(3)�١��ܡ��ݡ����е�ijЩԪ�ؿ��γɼȺ����Ӽ��ֺ����ۼ������ӻ����д������һ�ֻ�����ĵ���ʽ_______________��
(4)�ɱ�������Ԫ�ص�ԭ�Ӱ�1��1��ɵij���Һ̬�������ϡ��Һ�ױ����ֽ⣬�������ʲ������÷�Ӧ��������(�����)________��
a��MnO2����b�� CuSO4 c��Na2SO3 d��FeCl3
(5) W��������ڵ�ͬ����Ԫ�ء����±����г�H2WO3�ĸ��ֲ�ͬ��ѧ���ʣ�������д����Ӧ�Ļ�ѧ����ʽ��
|
��� |
���� |
��ѧ����ʽ |
|
ʾ�� |
������ |
H2WO3��3H3PO3===3H3PO4��H2W�� |
|
1 |
|
|
|
2 |
|
|
(6)�ɱ���Ԫ���γɵij�������X��Y��Z��M��N�ɷ������·�Ӧ��

X��Һ��Y��Һ��Ӧ�����ӷ���ʽ______________��
N���ĵ��ʵĻ�ѧ����ʽΪ____________��
M��Һ������Ũ���ɴ�С������˳����______________ ��
M�������ӵļ������� __________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com