
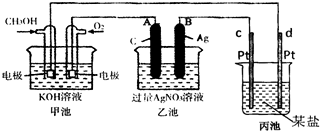
��ͼ��һ����ѧ���̵�ʾ��ͼ��
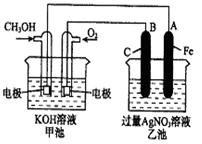
��֪�׳ص��ܷ�ӦʽΪ��2CH3OH+3O2+4KOH![]()
��1����ش�ͼ�мס������ص����ơ�
����� װ�ã��ҳ��� װ�á�
��2����ش����е缫�����ƣ�
ͨ��CH3OH�ĵ缫������ ��B��ʯī���缫�������� ��
��3��д���缫��Ӧʽ��
ͨ��O2�ĵ缫�ĵ缫��Ӧʽ�� ��A��Fe���缫�ĵ缫��ӦʽΪ
��
��4���ҳ��з�Ӧ�Ļ�ѧ����ʽΪ ��
��5�����ҳ���A��Fe��������������5.40gʱ���׳�������������O??2 mL����״���£�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
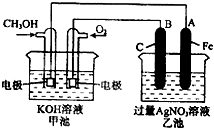 ��2012?��Ԫģ�⣩��ͼ��һ����ѧ���̵�ʾ��ͼ����֪�׳ص��ܷ�ӦʽΪ��2CH3OH+3O2+4KOH�T2K2CO3+6H2O
��2012?��Ԫģ�⣩��ͼ��һ����ѧ���̵�ʾ��ͼ����֪�׳ص��ܷ�ӦʽΪ��2CH3OH+3O2+4KOH�T2K2CO3+6H2O
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
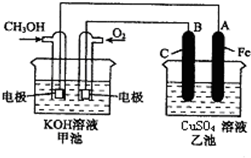 ��ͼ��һ����ѧ���̵�ʾ��ͼ����֪�׳ص��ܷ�ӦʽΪ��2CH3OH+3O2+4KOH�T2K2CO3+6H2O
��ͼ��һ����ѧ���̵�ʾ��ͼ����֪�׳ص��ܷ�ӦʽΪ��2CH3OH+3O2+4KOH�T2K2CO3+6H2O
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
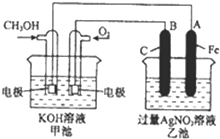 ��ͼ��һ����ѧ���̵�ʾ��ͼ����֪�׳ص��ܷ�ӦʽΪ��
��ͼ��һ����ѧ���̵�ʾ��ͼ����֪�׳ص��ܷ�ӦʽΪ��
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ͼ��һ����ѧ���̵�ʾ��ͼ���ش��������⣺
��ͼ��һ����ѧ���̵�ʾ��ͼ���ش��������⣺
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ͼ��һ����ѧ���̵�ʾ��ͼ��
��ͼ��һ����ѧ���̵�ʾ��ͼ���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com