
·ÖĪö £Ø1£©·“Ó¦ŗóČÜÖŹĪŖµČÅØ¶ČµÄ“×ĖįÄĘŗĶ“×Ėį£¬»ģŗĻŅŗµÄpH=6£¬ČÜŅŗĻŌĖįŠŌ£¬ĒāĄė×ÓÅضČĪŖc£ØH+£©=1”Į10-6mol/L£¬ŌņĒāŃõøłĄė×ÓÅضČĪŖ£ŗc£ØOH-£©=1”Į10-8mol/L£¬½įŗĻµēŗÉŹŲŗć½ųŠŠ¼ĘĖć£»
£Ø2£©øł¾Żc£Ø“ż²ā£©=$\frac{V£Ø±ź×¼£©”Įc£Ø±ź×¼£©}{V£Ø“ż²ā£©}$·ÖĪö²»µ±²Ł×÷¶ŌV£Ø±ź×¼£©”ĮµÄÓ°Ļģ£¬ŅŌ“ĖÅŠ¶ĻÅØ¶ČµÄĪó²ī£»
£Ø3£©ČōĖłµĆ»ģŗĻŅŗµÄpH=2£¬øł¾Żc£ØH+£©=$\frac{c£ØĖį£©V£ØĖį£©-c£Ø¼ī£©V£Ø¼ī£©}{V£ØĖį£©+V£Ø¼ī£©}$¼ĘĖćĖį¼īµÄĢå»ż±Č£®
½ā“š ½ā£ŗ£Ø1£©·“Ó¦ŗóČÜÖŹĪŖµČÅØ¶ČµÄ“×ĖįÄĘŗĶ“×Ėį£¬»ģŗĻŅŗµÄpH=6£¬ČÜŅŗĻŌĖįŠŌ£¬Ōņc£ØH+£©£¾c£ØOH-£©£¬ÓɵēŗÉŹŲŗćc£ØCH3COO-£©+c£ØOH-£©=c£ØNa+£©+c£ØH+£©æÉÖŖ£¬c£ØCH3COO-£©£¾c£ØNa+£©£¬ŌņČÜŅŗÖŠĮ£×ÓÅØ¶Č¹ŲĻµĪŖ£ŗc£ØCH3COO-£©£¾c£ØNa+£©£¾c£ØCH3COOH£©£¾c£ØH+£©£¾c£ØOH-£©£»ŅŃÖŖĒāĄė×ÓÅضČĪŖc£ØH+£©=1”Į10-6mol/L£¬ŌņĒāŃõøłĄė×ÓÅضČĪŖ£ŗc£ØOH-£©=1”Į10-8mol/L£¬øł¾ŻµēŗÉŹŲŗćæÉÖŖ£ŗc£ØCH3COO-£©-c£ØNa+£©=c£ØOH-£©-c£ØH+£©=1”Į10-6mol/L-1”Į10-8mol/L=9.9”Į10-7mol/L£»
¹Ź“š°øĪŖ£ŗc£ØCH3COO-£©£¾c£ØNa+£©£¾c£ØCH3COOH£©£¾c£ØH+£©£¾c£ØOH-£©£»9.9”Į10-7mol/L£»
£Ø2£©A£®ÅäÖʱź×¼ČÜŅŗµÄ¹ĢĢåNaOHÖŠ»ģÓŠKOHŌÓÖŹ£¬ŌņŌŚµĪ¶ØŹ±ĻūŗĵÄŃĪĖįĘ«ÉŁ£¬ĖłŅŌŃĪĖįµÄĢå»żŅ»¶ØŹ±£¬Ļūŗĵıź×¼ČÜŅŗµÄĢå»żĘ«“ó£¬Ōņ²ā¶Ø½į¹ūĘ«“󣬹ŹAÕżČ·£»
B£®µĪ¶ØÖÕµć¶ĮŹżŹ±£¬ŃöŹÓµĪ¶Ø¹ÜµÄæĢ¶Č£¬Ōņ±ź×¼ČÜŅŗµÄĢå»żĘ«“ó£¬Ōņ²ā¶Ø½į¹ūĘ«“󣬹ŹBÕżČ·£¬
C£®Ź¢×°Ī“ÖŖŅŗµÄ׶ŠĪĘæÓĆÕōĮóĖ®Ļ“¹żŗóŌŁÓĆĪ“ÖŖŅŗČóĻ“£¬×¶ŠĪĘæÖŠŃĪĖįµÄĪļÖŹµÄĮæĘ«“󣬵Ī¶ØŹ±Ļūŗĵıź×¼ČÜŅŗµÄĢå»żĘ«“ó£¬ĖłŅŌ²ā¶Ø½į¹ūĘ«“󣬹ŹCÕżČ·£»
D£®µĪ¶Øµ½ÖÕµć¶ĮŹżŹ±£¬·¢ĻÖµĪ¶Ø¹Ü¼ā×ģ“¦Šü¹ŅŅ»µĪČÜŅŗ£¬ŌņĻūŗĵıź×¼ČÜŅŗµÄĢå»żĘ«“ó£¬ĖłŅŌ²ā¶Ø½į¹ūĘ«“󣬹ŹDÕżČ·£»
¹Ź“š°øĪŖ£ŗABCD£»
£Ø3£©Ä³ĪĀ¶ČĻĀĖ®µÄĄė×Ó»żĪŖKW=1”Į10-13£¬ČōĖłµĆ»ģŗĻŅŗµÄpH=2£¬ŌņÓŠ$\frac{0.1b-0.01a}{a+b}$=0.01£¬½āµĆa£ŗb=9£ŗ2£¬
¹Ź“š°øĪŖ£ŗ9£ŗ2£®
µćĘĄ ±¾Ģāæ¼²éĖį¼ī»ģŗĻµÄ¶ØŠŌÅŠ¶Ļ”¢pHµÄÓŠ¹Ų¼ĘĖć”¢Ėį¼īÖŠŗĶµĪ¶ØĪó²ī·ÖĪöµČ£¬»įøł¾ŻČÜŅŗµÄĖį¼īŠŌ¼°µēŗÉŹŲŗćÅŠ¶ĻĄė×ÓÅØ¶Č“óŠ”£¬×¢Ņā£Ø1”¢3£©ÖŠĖ®µÄĄė×Ó»ż³£ŹżŹĒ10-13¶ų²»ŹĒ10-14£¬·ńŌņ»įµ¼ÖĀ“ķĪó£¬ĪŖŅדķµć£»²ąÖŲÓŚæ¼²éѧɜ¶Ō»ł“”ÖŖŹ¶µÄÓ¦ÓĆÄÜĮ¦ŗĶ¼ĘĖćÄÜĮ¦£®


 ĆūŠ£æĪĢĆĻµĮŠ“š°ø
ĆūŠ£æĪĢĆĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
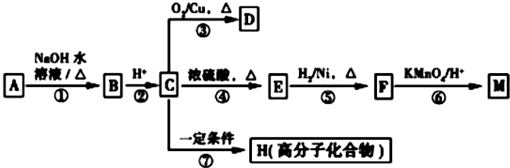
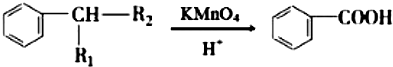 £ØR1”¢R2±ķŹ¾Ģž»ł»ņĒāŌ×Ó£©
£ØR1”¢R2±ķŹ¾Ģž»ł»ņĒāŌ×Ó£©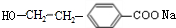 £®
£®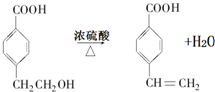 £®
£®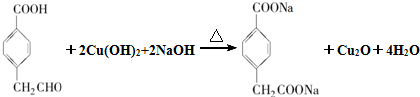 £®
£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
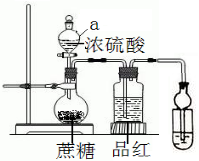 ŌŚÉÕĘæ·Å20æĖÕįĢĒ£¬ŅĄ“Ī¼ÓČėÉŁĮæĖ®”¢20mLÅØĮņĖį£¬ÕįĢĒÖš½„±äŗŚ£¬Ģå»żÅņÕĶ£¬ŠĪ³ÉŹčĖɶąæ×µÄŗŚĆę°ü£¬ÉÕĘæ·¢ĢĢ£¬Ę·ŗģČÜŅŗŃÕÉ«±äµ£®Ēė»Ų“š£ŗ
ŌŚÉÕĘæ·Å20æĖÕįĢĒ£¬ŅĄ“Ī¼ÓČėÉŁĮæĖ®”¢20mLÅØĮņĖį£¬ÕįĢĒÖš½„±äŗŚ£¬Ģå»żÅņÕĶ£¬ŠĪ³ÉŹčĖɶąæ×µÄŗŚĆę°ü£¬ÉÕĘæ·¢ĢĢ£¬Ę·ŗģČÜŅŗŃÕÉ«±äµ£®Ēė»Ų“š£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĶʶĻĢā

 £®
£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ÓĆĻū¶¾ŅŗɱƚÓĪÓ¾³ŲÖŠµÄŌåĄą | |
| B£® | ÓĆCaCl2ČŚ»ÆĀ·ĆęµÄ»żŃ© | |
| C£® | ÓĆŹÆ»ŅŠŽø“±»ĖįÓź½žŹ“µÄĶĮČĄ | |
| D£® | ÓĆ“ß»Æ¼Į½«Ęū³µĪ²ĘųÖŠµÄCOŗĶNO×Ŗ»ÆĪŖĪŽŗ¦ĪļÖŹ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ³£ĪĀĻĀ0.4 mol/L HBČÜŅŗŗĶ 0.2 mol/L NaOHČÜŅŗµČĢå»ż»ģŗĻŗóČÜŅŗµÄpH=3£¬Ōņ»ģŗĻČÜŅŗÖŠĄė×ÓÅØ¶ČµÄ“óŠ”Ė³ŠņĪŖ£ŗc£ØB-£©£¾c£ØH+£©£¾c£ØNa+£©£¾c£ØOH-£© | |
| B£® | µČÅØ¶ČµÄĻĀĮŠĻ”ČÜŅŗ£ŗ¢ŁĮņĖįĒāÄĘ ¢ŚŅŅĖįÄĘ ¢Ū“×Ėį ¢ÜĢ¼ĖįĒāÄĘ ¢ŻĻõĖįÄĘ ¢Ž±½·ÓÄĘ£¬ĖüĆĒµÄpHÓÉŠ”µ½“óÅÅĮŠĪŖ£ŗ¢Ū¢Ż¢Ł¢Ü¢Ś¢Ž | |
| C£® | ³£ĪĀĻĀ0.1 mol/LµÄĻĀĮŠČÜŅŗ ¢ŁNH4Al£ØSO4£©2 ¢ŚNH4Cl ¢ŪNH3•H2O ¢ÜCH3COONH4ÖŠc £ØNH4+£©Óɓ󵽊”µÄĖ³ŠņŹĒ£ŗ¢Ś£¾¢Ł£¾¢Ü£¾¢Ū | |
| D£® | ŌŚ25”ꏱ£¬½«a mol•L-1µÄ°±Ė®Óė0.01 mol•L-1µÄŃĪĖįµČĢå»ż»ģŗĻ·“Ó¦Ź±ČÜŅŗÖŠc£ØNH4+£©=c£ØCl-£©£®ÓĆŗ¬aµÄ“śŹżŹ½±ķŹ¾NH3•H2OµÄµēĄė³£ŹżKb=$\frac{1{0}^{-9}}{a-0.01}$ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com