
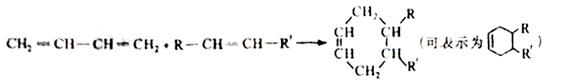
 �к��������ŵ�������________________��
�к��������ŵ�������________________�� ����ԭ�Ӳ���̼̼˫����̼̼����ֱ������������״�ṹ��C���ʵ�����ͬ���칹��Ľṹ��ʽ��_____________��
����ԭ�Ӳ���̼̼˫����̼̼����ֱ������������״�ṹ��C���ʵ�����ͬ���칹��Ľṹ��ʽ��_____________�� �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
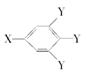 (���У���X����Y��Ϊ������)��
(���У���X����Y��Ϊ������)���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 2CH3COOH��
2CH3COOH��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
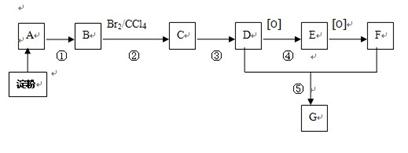
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
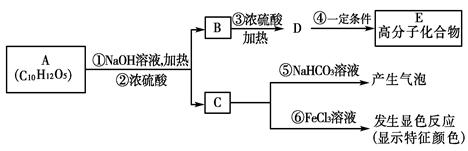
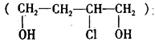 �Ǻϳɸ߷��ӻ�����HPMA���м��壬HPMA�����ڹ�¯�蹸����
�Ǻϳɸ߷��ӻ�����HPMA���м��壬HPMA�����ڹ�¯�蹸����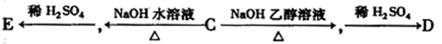
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������


| A��������Ӧ | B���ӳɷ�Ӧ | C��ȡ����Ӧ | D��ˮ�ⷴӦ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

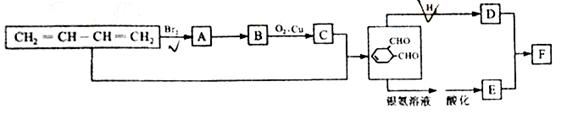
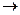 C�ķ�Ӧ����ʽ��
C�ķ�Ӧ����ʽ��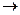 F�ķ�Ӧ����Ϊ________________________��
F�ķ�Ӧ����Ϊ________________________��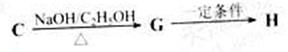
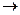 G�ķ�Ӧ����Ϊ_____________________.
G�ķ�Ӧ����Ϊ_____________________.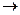 H�ķ�Ӧ����ʽ��_______________________��
H�ķ�Ӧ����ʽ��_______________________���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
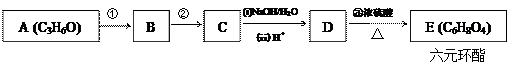
�鿴�𰸺ͽ���>>
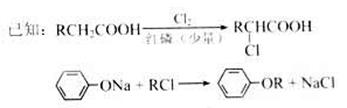
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 �ʵĽṹ��ʽ�ֱ�Ϊ��
�ʵĽṹ��ʽ�ֱ�Ϊ���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com