

(1)��Щװ�õ�����˳��(���������ҵķ���)��_________��_________��_________�� _________��_________��_________��_________��__________(����ӿڵı��)��
(2)ʵ��ʱ��������������ԭ����_________________________________________________��
(3)���Ҵ�����ͨ��O2��ΪʹSO2�нϸߵ�ת���ʣ�ʵ��ʱ����Ũ��������ȴ������Ⱥ�˳����____________��
(4)�����۲쵽��������_____________________________________________��
(5)��n mol Na2SO3��ĩ������Ũ������д�ʵ�飬����Ӧ����ʱ������ͨ��O2һ��ʱ��Ƶâ�����m g����ʵ����SO2��ת����Ϊ___________��
�������������ȡ������Ҫ���ǵ����������ӡ���ȡ��Ӧ���ռ���β�����������ԣ�װ�õ�����˳����a h i b c f g d���ڶ�����ѹǿԭ����Ӧ�á��ش�����ʱ��һ���μ�ʵ��Ŀ�ģ��Ͳ��ѻش����⡣
�𰸣�(1)a h i b c f g d
(2)������ʹŨ������˳���ص�����ƿ�У�ԭ����ά����ƿ��ѹǿ���Һ©����ѹǿ���
(3)�ȼ���V2O5��������Ũ����
(4)����ɫ(���ɫ)����(�����)����
(5)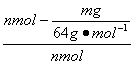 ��100%��
��100%��![]() ��100%
��100%


 �㽭֮��ѧҵˮƽ����ϵ�д�
�㽭֮��ѧҵˮƽ����ϵ�д� ��Ч���ܿ�ʱ��ҵϵ�д�
��Ч���ܿ�ʱ��ҵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ʵ������ȡ��ϩ�ķ�Ӧԭ��ΪCH3CH2OH
ʵ������ȡ��ϩ�ķ�Ӧԭ��ΪCH3CH2OH
| ||
| 170�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �ɶ�����Ԫ����ɵ�10������A��J֮������ͼ��ϵ����֪��A��BΪͬ��������Ԫ�صĵ��ʣ������Ϊ�����ͨ�������AΪ���壬B��DΪ������B�ʻ���ɫ��FΪҺ�壬A��G��Ũ��Һ����ʱ��Ӧ����D��F��J�ڹ���ʱ��I���ɣ�
�ɶ�����Ԫ����ɵ�10������A��J֮������ͼ��ϵ����֪��A��BΪͬ��������Ԫ�صĵ��ʣ������Ϊ�����ͨ�������AΪ���壬B��DΪ������B�ʻ���ɫ��FΪҺ�壬A��G��Ũ��Һ����ʱ��Ӧ����D��F��J�ڹ���ʱ��I���ɣ�

 MnCl2+Cl2��+2H2O
MnCl2+Cl2��+2H2O MnCl2+Cl2��+2H2O
MnCl2+Cl2��+2H2O| ʵ�鲽�� | ʵ������ | �û�ѧ������� |
| ����������ͨ �����KI��Һ |
��Һ������ �� �� ɫ |
Cl2+2I-=2Cl-+I2 Cl2+2I-=2Cl-+I2 �������ӷ���ʽ��ʾ�� |
| ����ͨ������ | ��Һ�����ɫ | 5Cl2+I2+6H2O=10HCl+2HIO3 5Cl2+I2+6H2O=10HCl+2HIO3 ���û�ѧ����ʽ��ʾ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(10��)ʵ������ȡ��ϩ�ķ�Ӧԭ��Ϊ,��Ӧʱ�������¶ȹ��߶�ʹ�Ҵ���ŨH2SO4��Ӧ����������SO2�������������ʵ��ȷ�������������������ϩ�Ͷ�������
�Իش��������⣺
(1)ͼ�Т٢ڢۢ�װ��ʢ�ŵ��Լ��ֱ���(����)����________����________����________����________��
A��Ʒ����Һ B��NaOH��Һ C��Ũ���� D�����Ը��������Һ
(2)��˵��SO2���ڵ�������______________________________________________��
(3)ʹ��װ�âڵ�Ŀ����________________________________________________��
(4)ʹ��װ�â۵�Ŀ����__________________________________________________��
(5)ȷ֤��ϩ���ڵ�������__________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014���Ĵ�ʡ�����и߶���ѧ����ĩ��⻯ѧ�Ծ��������棩 ���ͣ�ʵ����
ʵ������ȡ��ϩ�ķ�Ӧԭ��Ϊ��CH3CH2OH CH2=CH2����H2O����Ӧʱ�������¶ȹ��߶�ʹ�Ҵ���ŨH2SO4��Ӧ����������SO2�������������ʵ��ȷ�������������������ϩ�Ͷ�������
CH2=CH2����H2O����Ӧʱ�������¶ȹ��߶�ʹ�Ҵ���ŨH2SO4��Ӧ����������SO2�������������ʵ��ȷ�������������������ϩ�Ͷ�������

�Իش��������⣺
��1��ͼ�Т٢ڢۢ�װ��ʢ�ŵ��Լ��ֱ��ǣ���ѡ����ĸ������_________����_________��
A��Ʒ����Һ B��NaOH��Һ C��Ũ���� D�����Ը��������Һ
��2����˵��SO2���ڵ�������___________________________________________________��
��3��ȷ֤��ϩ���ڵ�������_______________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013������ʡ����Э�����һ��ѧ�����п��Ի�ѧ�Ծ� ���ͣ�ʵ����
(10��)ʵ������ȡ��ϩ�ķ�Ӧԭ��Ϊ ,
��Ӧʱ�������¶ȹ��߶�ʹ�Ҵ���ŨH2SO4��Ӧ����������SO2�������������ʵ��ȷ�������������������ϩ�Ͷ�������
,
��Ӧʱ�������¶ȹ��߶�ʹ�Ҵ���ŨH2SO4��Ӧ����������SO2�������������ʵ��ȷ�������������������ϩ�Ͷ�������

�Իش��������⣺
(1)ͼ�Т٢ڢۢ�װ��ʢ�ŵ��Լ��ֱ���(����)����________����________����________����________��
A��Ʒ����Һ B��NaOH��Һ C��Ũ���� D�����Ը��������Һ
(2)��˵��SO2���ڵ�������______________________________________________��
(3)ʹ��װ�âڵ�Ŀ����________________________________________________��
(4)ʹ��װ�â۵�Ŀ����__________________________________________________��
(5)ȷ֤��ϩ���ڵ�������__________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com