

| ||
| ||


 ½ĢÓżŹĄ¼ŅדŌŖ¾ķĻµĮŠ“š°ø
½ĢÓżŹĄ¼ŅדŌŖ¾ķĻµĮŠ“š°ø »ĘøŌæĪĢĆ×÷Ņµ±¾ĻµĮŠ“š°ø
»ĘøŌæĪĢĆ×÷Ņµ±¾ĻµĮŠ“š°ø µ„ŌŖ¼ÓĘŚÄ©ø“Ļ°ĻČ·ę“óæ¼¾ķĻµĮŠ“š°ø
µ„ŌŖ¼ÓĘŚÄ©ø“Ļ°ĻČ·ę“óæ¼¾ķĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
 £Ø2011?Äž²ØÄ£Äā£©”°»ÆѧÓė¼¼Źõ”±Ä£æé
£Ø2011?Äž²ØÄ£Äā£©”°»ÆѧÓė¼¼Źõ”±Ä£æé
| ||
| ||
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| ³É·Ö | Na+ | K+ | Ca2+ | Mg2+ | Cl- | SO 42- | HCO 3+ | ŗ¬Įæ/mg?L-1 | 9360 | 83 | 200 | 1100 | 16000 | 1200 | 118 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗɽ¶«Ż·ĻŲŹµŃéøßÖŠ2010½ģøßČżæ¼Ē°Č«ÕęÄ£Äā£ØĄķ×Ū»Æѧ²æ·Ö£© ĢāŠĶ£ŗĢīæÕĢā
£Ø8·Ö£©”¾»Æѧ-»ÆѧÓė¼¼Źõ”æ
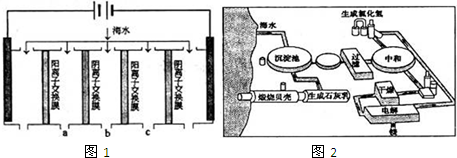
ŗ£ŃóŹĒ»Æѧ׏Ō“±¦æā£¬ŗ£Ńó׏Ō“µÄ×ŪŗĻĄūÓĆ¾ßÓŠ¹ćĄ«µÄĒ°¾°”£»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ÕōĮó·ØŹĒČĖĄą×īŌēŹ¹ÓƵĵ»Æŗ£Ė®µÄ·½·Ø£¬¼¼ŹõŗĶ¹¤ŅÕ±Č½ĻĶź±ø£¬µ«³£¹ęÕōĮó·Ø·ØŅ²“ęŌŚ½Ļ“óȱĻŻ£¬ĒėĖµĆ÷³£¹ęÕōĮó·Øµ»Æŗ£Ė®µÄ×ī“óȱĻŻ”£
__________________________________________________________
£Ø2£©Ąė×Ó½»»»·ØŅ²ŹĒ³£ÓƵÄĖ®“¦Ąķ¼¼Źõ”£¾Ū±ūĻ©ĖįÄĘŹĒŅ»ÖÖĄė×Ó½»»»Ź÷Ö¬£¬Š“³ö¾Ū±ūĻ©ĖįÄʵ„ĢåµÄ½į¹¹¼ņŹ½ ”£
£Ø3£©ÄæĒ°“Óŗ£Ė®ÖŠĢįČ”äåµÄ³£ÓĆĮ÷³ĢČēĻĀ£ØæąĀ±£¬ŗ£Ė®Õō·¢½į¾§·ÖĄė³öŹ³ŃĪµÄÄøŅŗ£©£ŗ

“Ó·“Ó¦¢ŪŗóµÄČÜŅŗÖŠ·ÖĄė³öµ„ÖŹäåµÄ·½·ØŹĒ ”£
£Ø4£©ŗ£Ė®ĢįĆ¾¹ż³ĢÖŠ£¬ÓėŹÆ»ŅŅ¤ŗĶŃĪĖį³§ÓŠ×ŽōĆܵÄĮŖĻµ£¬ŹŌĖµĆ÷ŗ£Ė®ĢįĆ¾ÓėŃĪĖį³§Ö®¼äµÄ±ŲČ»ĮŖĻµ”£__________________________________________________________
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011ÄźÕć½Ź”Äž²ØŹŠøßæ¼»ÆŃ§Ä£ÄāŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗ½ā“šĢā

²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com