
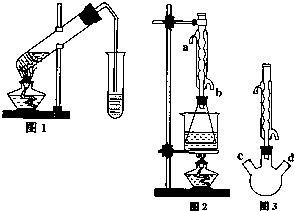
 ������������Ҫ�Ĺ�ҵԭ�Ϻ��ܼ���ͨ�����������Ҵ������ᣮijѧϰС������ͼ1װ����ȡ���������ֲ�Ʒ���ٷ������������ĺ�����
������������Ҫ�Ĺ�ҵԭ�Ϻ��ܼ���ͨ�����������Ҵ������ᣮijѧϰС������ͼ1װ����ȡ���������ֲ�Ʒ���ٷ������������ĺ�����| �������� | �Ҵ� | ���� | |
| �е� | 77.1�� | 78.5�� | 117.9�� |
| 15.84g |
| 20g |


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ������Ҫ���л��ϳ��м��壬�㷺Ӧ�����ܼ������ܼ������ϡ�ճ�ϼ���ӡˢ����֯�ȹ�ҵ������������ʵ���Һ�ҵ�Ʒ����������·�Ӧ��CH3COOH+C2H5OH
������Ҫ���л��ϳ��м��壬�㷺Ӧ�����ܼ������ܼ������ϡ�ճ�ϼ���ӡˢ����֯�ȹ�ҵ������������ʵ���Һ�ҵ�Ʒ����������·�Ӧ��CH3COOH+C2H5OH | Ũ���� | �� |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ���� | ������ɫ��̬ | �ܶȣ�g/ml�� | �۵㣨�棩 | �е㣨�棩 |
| ������ | ��ɫ���� | 1.2659 | 122 | 249 |
| �״� | ��ɫҺ�� | 0.7915 | -97.8 | 64.65 |
| ��������� | ��ɫҺ�� | 1.0888 | -12.3 | 199.6 |
| ���� | ��ɫҺ�� | \ | 16.6 | 117.9 |
| �Ҵ� | ��ɫҺ�� | \ | -117.3 | 78.5 |
| �������� | ��ɫҺ�� | \ | 83.6 | 77.1 |

| ŨH2SO4 |
| �� |


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2009���Ϻ����ϻ���ѧ�����꼶����¿�����ѧ�Ծ� ���ͣ�058
������������Ҫ�Ĺ�ҵԭ�Ϻ��ܼ�����������ͼװ����ȡ���������Ĵֲ�Ʒ���ٲⶨ���������ĺ�����

(1)д����Ӧ����ʽ________(��Ӧ)
(2)ʵ����һ���ñ���̼������Һ���շ�Ӧ��������������Թܣ��ɿ�����������________�������Թܣ��ɿ�����������________��������ȴ�����˰�ɫ���壬�þ�����________��
(3)��������������������װ�ã�Ϊ����߲��ʣ������һ���Ľ������
________��
(4)Ϊ�ⶨ���������ĺ������������²�����
(��)ȷ����20.0 g����������Ʒ����ƿ�У���0.50 mol/L��NaOH�ζ�(��̪��ʾ��)���յ�ʱ����NaOH��Һ�����Ϊ40.0 mL
(��)��ȡ20.0 g��������ֲ�Ʒ��250 mL��ƿ�У�����100 mL��2.1 mol/L��NaOH��Һ�����ɫ�Ⱥ�װ�������ܣ���ˮԡ�ϼ��Ȼ���Լ1Сʱ��װ����ͼ��ʾ������ȴ����0.50 mol/LHC1�ζ�������NaOH���յ�ʱ������������Ϊ20.0 mL��
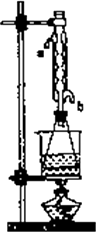
�ش𣺢�ʵ��(��)�дﵽ�ζ��յ�ʱ��������________��
��ʵ��(��)����ˮ����ˮ���ܵ�________(��a��b)�ܿ�ͨ�룮
������ʵ��(��)��(��)���������ݼ���ֲ�����������������������Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 (08�Ϻ��ɽ���ģ��)������������Ҫ�Ĺ�ҵԭ�Ϻ��ܼ�����������ͼ
(08�Ϻ��ɽ���ģ��)������������Ҫ�Ĺ�ҵԭ�Ϻ��ܼ�����������ͼ
װ����ȡ���������ֲ�Ʒ���ٲⶨ���������ĺ�����
��д���˷�Ӧ�Ļ�ѧ����ʽ
��
�÷�ӦҪ��Ũ������ڵ������½��У�Ũ�����������
��
��ʵ����һ���ñ���̼������Һ���շ�Ӧ�����������
���Թܣ��ɿ����������� ������
�����Թܣ��ɿ����������� ������ʱȴ�����˰�ɫ���壬�þ����� ��
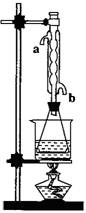 �Ƕ�������������������װ�ã�Ϊ����߲��ʣ������
�Ƕ�������������������װ�ã�Ϊ����߲��ʣ������
һ���Ľ������
��
��Ϊ�ⶨ���������ĺ��������������²�����
��I��ȷ����20.0g����������Ʒ����ƿ�У���0.50mol/L
NaOH�ζ�����̪��ָʾ�������յ�ʱ����NaOH��Һ�����Ϊ
40.0mL��
��II����ȡ20.0g���������ֲ�Ʒ��250mL��ƿ�У�����100mL
2.1mol/LNaOH��Һ��Ͼ��Ⱥ�װ�������ܣ���ˮԡ�ϼ��Ȼ�
��Լ1Сʱ��װ����ͼ��ʾ������ȴ����0.50mol/LHCl�ζ�
������NaOH���յ�ʱ������������Ϊ20.0mL��
�ش�
��ʵ�飨I���дﵽ�ζ��յ�ʱ�������� ��
��ʵ�飨II������ˮ����ˮ���ܵ� ����a��b���ܿ�ͨ�롣
������ʵ�飨I������II�����������ݼ���ֲ�����������������������Ϊ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com