
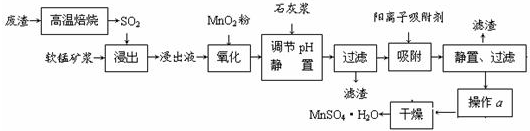
����SO2���ŷš���������SO2��Ϊ�����Ե��о����⡣�ҹ��о���Ա���Ƶ����õ�Ʒλ���̿�(��Ҫ�ɷ���MnO2)���շ������±��ղ�����SO2���Ʊ������̵������������£�

����Һ��pH��2�����еĽ���������Ҫ��Mn2����������������Fe2����Al3����Ca2����Pb2���������������ӡ�
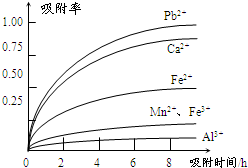
�йؽ������ӵİ뾶�Լ��γ������������ʱ��pH���±��������������������������ӵ�Ч������ͼ��

| ���� | ���Ӱ뾶(pm) | ��ʼ����ʱ��pH | ��ȫ����ʱ��pH |
| Fe2�� | 74 | 7.6 | 9.7 |
| Fe3�� | 64 | 2.7 | 3.7 |
| Al3�� | 50 | 3.8 | 4.7 |
| Mn2�� | 80 | 8.3 | 9.8 |
| Pb2�� | 121 | 8.0 | 8.8 |
| Ca2�� | 99 | �� | �� |
��֪PbO2�������Դ���MnO2����ش��������⣺
�� д��������������Ҫ��Ӧ�Ļ�ѧ����ʽ ��������������Ҫ��Ӧ�����ӷ���ʽ ��
�� ���������Һ���м���ʯ�ҽ������ڵ���pH��pHӦ������ ��
�� ���������������ڳ�ȥ���ʽ������ӡ���������������������Ч���������� ����д��ţ���
a����Һ��pH b���������ӵĵ�� c���������ӵİ뾶 d������ʱ��
�� ����a���� �ȹ��̡�
�� SO2��MnO2��MnSO4 2Fe2����MnO2��4H����2Fe3����Mn2����2H2O
�� 4.7��8.3
�� b c d
�� ����Ũ���ᾧ
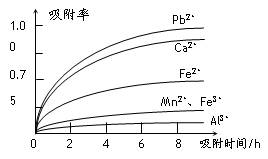
�������Ʊ������̵���������Ϊ֪ʶ���壬���黯ѧ��Ӧ����д���������е����⡣��1��������ͼ�����������������̿�MnO2����SO2�ķ�Ӧ������������ֻ��Fe2�����л�ԭ�ԣ����Ա�MnO2������������������Fe3������2�������к���Fe2����Al3����Ca2����Pb2�����������ӣ��ɳ�����pH��Χ֪��Fe2���ij�����Mn2�����ӵij��������pH�ӽ�����Fe3��������Զ���ʿ��Խ�Fe2��������Fe3�������ӡ��������ʵ�ͼ���Կ�����Ca2����Pb2���������ʽϸߣ���ֻҪ����pHֵ��4.7��8.3�䣬����4.7���Խ�Fe3����Al3����ȥ��С��8.3�Ƿ�ֹMn2��Ҳ��������3����ϰ뾶��������ͼ֪��ͼ�����Ӵ������£��뾶�м�С���ƣ���Ӧ�������ʼ�С������ʱ��ĵ������������ӵ������ʾ�������Fe3����Al3������������������������ʵ͡���4��������ȡ��MnSO4��H2O���нᾧˮ���ʲ�������Ũ���ᾧ�ķ�����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ���� | ���Ӱ뾶��pm�� | ��ʼ����pH | ��ȫ����pH |
| Fe2+ | 74 | 7.6 | 9.7 |
| Fe3+ | 64 | 2.7 | 3.7 |
| Al3+ | 50 | 3.8 | 4.7 |
| Mn2+ | 80 | 8.3 | 9.8 |
| Pb2+ | 121 | 8.0 | 8.8 |
| Ca2+ | 99 | - | - |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� | ���Ӱ뾶��pm�� | ��ʼ����ʱ��pH | ��ȫ����ʱ��pH |
| Fe2+ | 74 | 7.6 | 9.7 |
| Fe3+ | 64 | 2.7 | 3.7 |
| Al3+ | 50 | 3.8 | 4.7 |
| Mn2+ | 80 | 8.3 | 9.8 |
| Pb2+ | 121 | 8.0 | 8.8 |
| Ca2+ | 99 | - | - |
 ��֪PbO2�������Դ���MnO2����ش��������⣺
��֪PbO2�������Դ���MnO2����ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
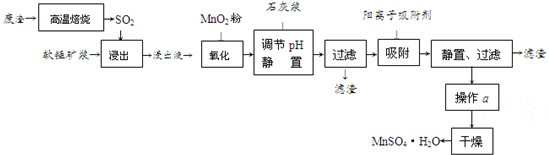
����SO2���ŷš���������SO2�ǻ�������Ҫ���⡣�ҹ��о���Ա���Ƶ����õ�Ʒλ���̿�(��Ҫ�ɷ���MnO2)���շ������±��ղ�����SO2���Ʊ������̵������������£�

����Һ��pH��2�����еĽ���������Ҫ��Mn2����������������Fe2����Al3����Ca2����Pb2���������������ӡ�
�йؽ������ӵİ뾶�Լ��γ������������ʱ��pH���±��������������������������ӵ�Ч������ͼ��
| ���� | ���Ӱ뾶(pm) | ��ʼ����ʱ��pH | ��ȫ����ʱ��pH |
| Fe2�� | 74 | 7.6 | 9.7 |
| Fe3�� | 64 | 2.7 | 3.7 |
| Al3�� | 50 | 3.8 | 4.7 |
| Mn2�� | 80 | 8.3 | 9.8 |
| Pb2�� | 121 | 8.0 | 8.8 |
| Ca2�� | 99 | �� | �� |
��֪PbO2��������ǿ��MnO2����ش��������⣺
�� д��������������Ҫ��Ӧ�Ļ�ѧ����ʽ ��
������������Ҫ��Ӧ�����ӷ���ʽ ��
�� ���������Һ���м���ʯ�ҽ������ڵ���pH��pHӦ��������Χ ��
��������Ҫ�ɷ��� ��
�� ���������������ڳ�ȥ���ʽ������ӡ���������������������Ч����������
����д��ţ���
a����Һ��pH b���������ӵ������� c���������ӵİ뾶 d������ʱ��
�� ����a���� ���̡�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2009��ɽ��ʡ�ij�������һ�и߿���ѧģ���Ծ����ţ��������棩 ���ͣ������

| ���� | ���Ӱ뾶��pm�� | ��ʼ����ʱ��pH | ��ȫ����ʱ��pH |
| Fe2+ | 74 | 7.6 | 9.7 |
| Fe3+ | 64 | 2.7 | 3.7 |
| Al3+ | 50 | 3.8 | 4.7 |
| Mn2+ | 80 | 8.3 | 9.8 |
| Pb2+ | 121 | 8.0 | 8.8 |
| Ca2+ | 99 | - | - |

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com